Question: Consider the reaction: O_2(g) ightleftharpoons 2O(g). Use the data below to estimate Delta S^o_{rxn} (in J/K): Select an answer and submit. For keyboard navigation, use
Consider the reaction: O_2(g) ightleftharpoons 2O(g). Use the data below to estimate \Delta S^o_{rxn} (in J/K): Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a -117 J/K b -498 J/K c 498 J/K d 117 J/K
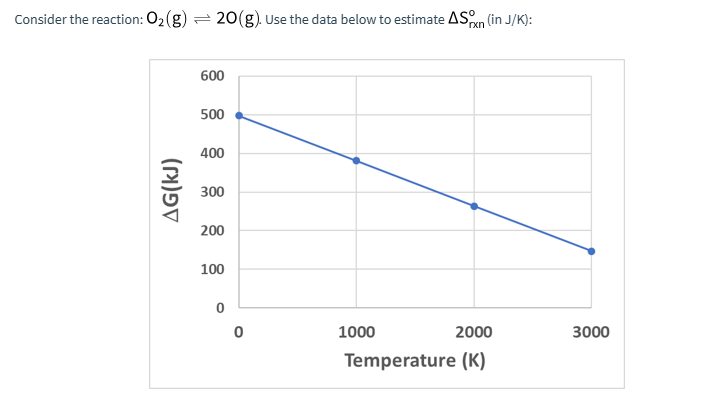 Consider the reaction: 02 (g) - 20(g) Use the data below to estimate AS (in J/K): 600 500 400 AG(KJ) 300 200 100 0 0 1000 2000 3000 Temperature (K)
Consider the reaction: 02 (g) - 20(g) Use the data below to estimate AS (in J/K): 600 500 400 AG(KJ) 300 200 100 0 0 1000 2000 3000 Temperature (K)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


