Question: Consider the reactive process. Performing a(n) would yield the following equation. (Note: this equation is shown without proper units.) (0.55)(100)(10)+(0.45)(100)(1)=(0.31)(65)(10)+(0.15)(65)(1)+(0.54)(65)(11) molecular H2 balance molecular C5H10
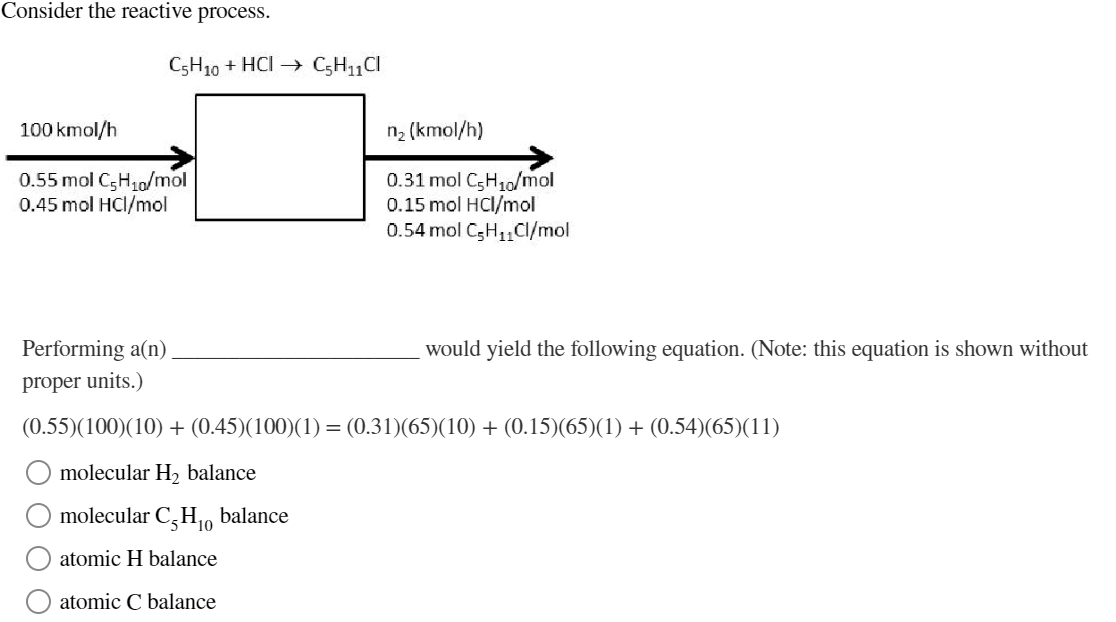
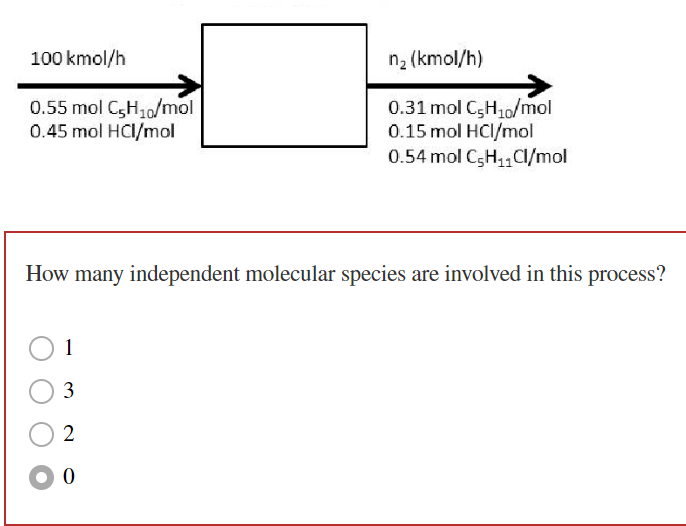
Consider the reactive process. Performing a(n) would yield the following equation. (Note: this equation is shown without proper units.) (0.55)(100)(10)+(0.45)(100)(1)=(0.31)(65)(10)+(0.15)(65)(1)+(0.54)(65)(11) molecular H2 balance molecular C5H10 balance atomic H balance atomic C balance How many independent molecular species are involved in this process? 1 3 2 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


