Question: Consider the redox reaction Fe3+ + V2+ Fe2+ + V3+ Iron Vanadium (a) Identify the oxidizing agent of the reaction. (b) Identify the reducing agent
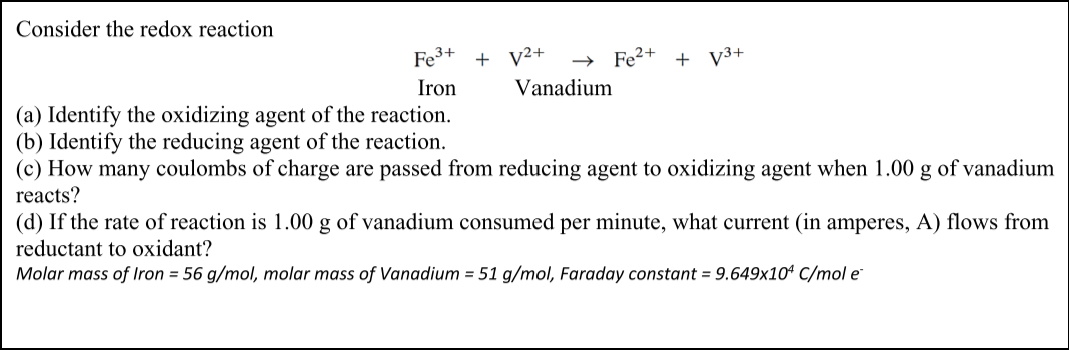
Consider the redox reaction Fe3+ + V2+ Fe2+ + V3+ Iron Vanadium (a) Identify the oxidizing agent of the reaction. (b) Identify the reducing agent of the reaction. (c) How many coulombs of charge are passed from reducing agent to oxidizing agent when 1.00 g of vanadium reacts? (d) If the rate of reaction is 1.00 g of vanadium consumed per minute, what current (in amperes, A) flows from reductant to oxidant? Molar mass of Iron = 56 g/mol, molar mass of Vanadium = 51 g/mol, Faraday constant = 9.649x104 C/mol e
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


