Question: Consider the reversible reaction A(g) = B(g) Which K values would indicate that there is more B than A at equilibrium? OK = 9 x
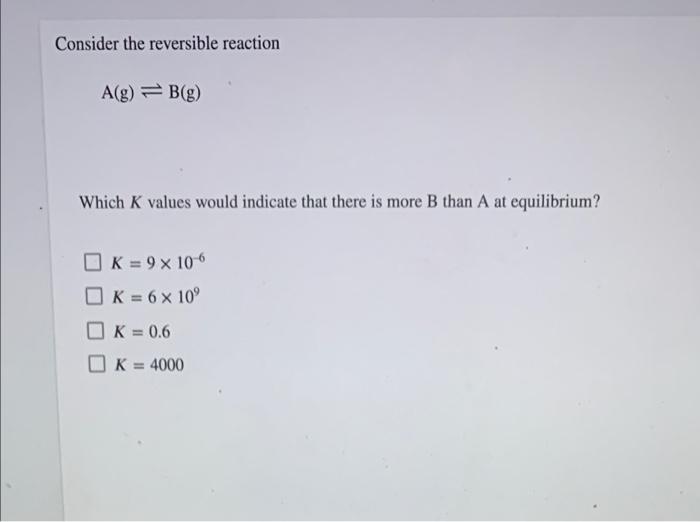
Consider the reversible reaction A(g) = B(g) Which K values would indicate that there is more B than A at equilibrium? OK = 9 x 10-6 O K = 6 x 10 K = 0.6 K = 4000
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


