Question: Consider the reversible, second-order reaction A+B A+B=C r = k1CACB - k-1CC = - k-1 (a) Solve the deterministic material balance for a constant-volume batch
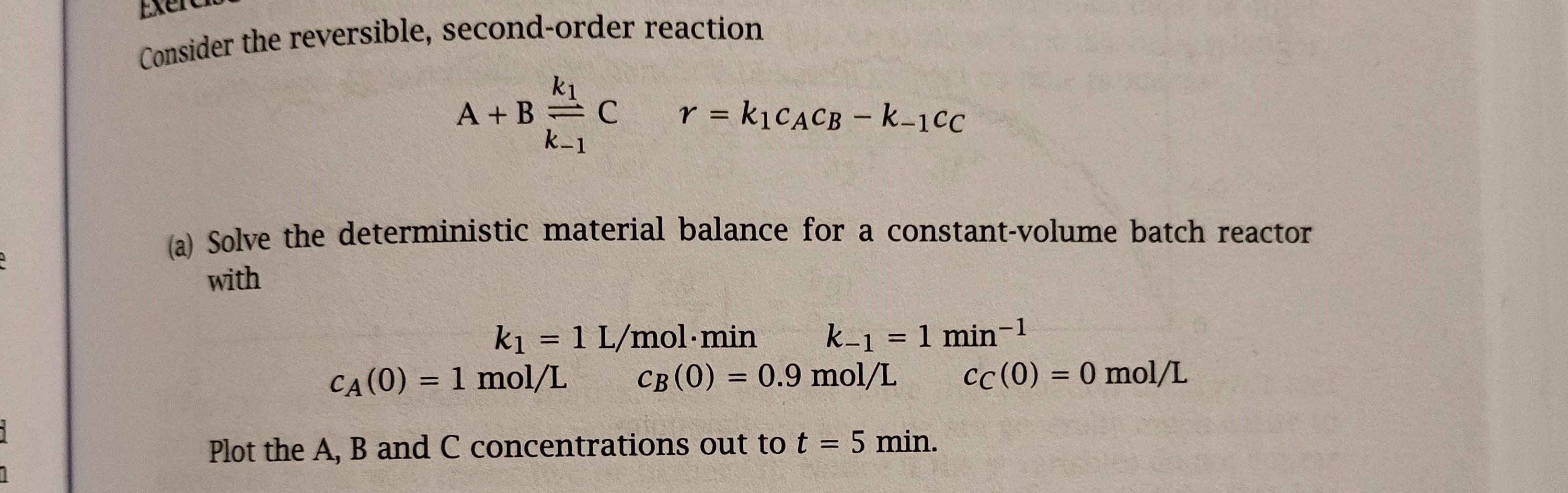
Consider the reversible, second-order reaction A+B A+B=C r = k1CACB - k-1CC = - k-1 (a) Solve the deterministic material balance for a constant-volume batch reactor with = k = 1 L/mol.min k-1 = 1 min-1 CA(0) = 1 mol/L CB (0) = 0.9 mol/L CC(0) = 0 mol/L = Plot the A, B and C concentrations out to t = 5 min. 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


