Question: Consider the six hypothetical electron states listed in the table. n A 3 B 3 1 1 D 2 1 C30 2 m F
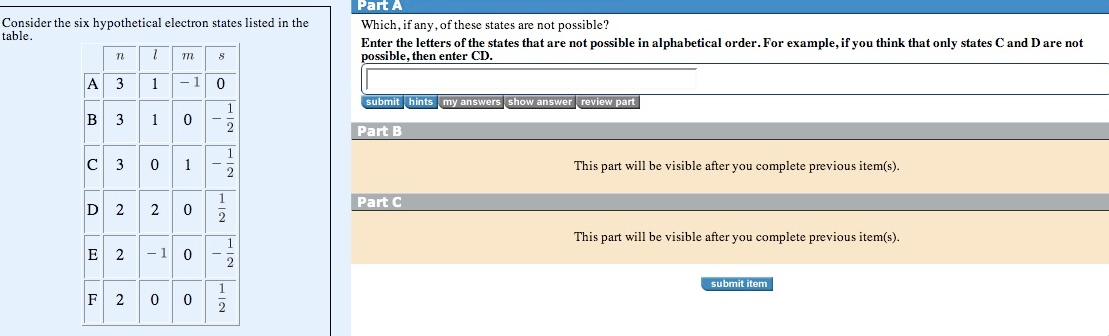
Consider the six hypothetical electron states listed in the table. n A 3 B 3 1 1 D 2 1 C30 2 m F 2 0 -1 0 0 1 0 E 2 - 1 0 8 0 - 1 2 i 1 2 1 2 2 1 1 MIT 2 Part A Which, if any, of these states are not possible? Enter the letters of the states that are not possible in alphabetical order. For example, if you think that only states C and D are not possible, then enter CD. submit hints my answers show answer review part Part B Part C This part will be visible after you complete previous item(s). This part will be visible after you complete previous item(s). submit item
Step by Step Solution
3.40 Rating (156 Votes )
There are 3 Steps involved in it
Answer Part A ... View full answer

Get step-by-step solutions from verified subject matter experts


