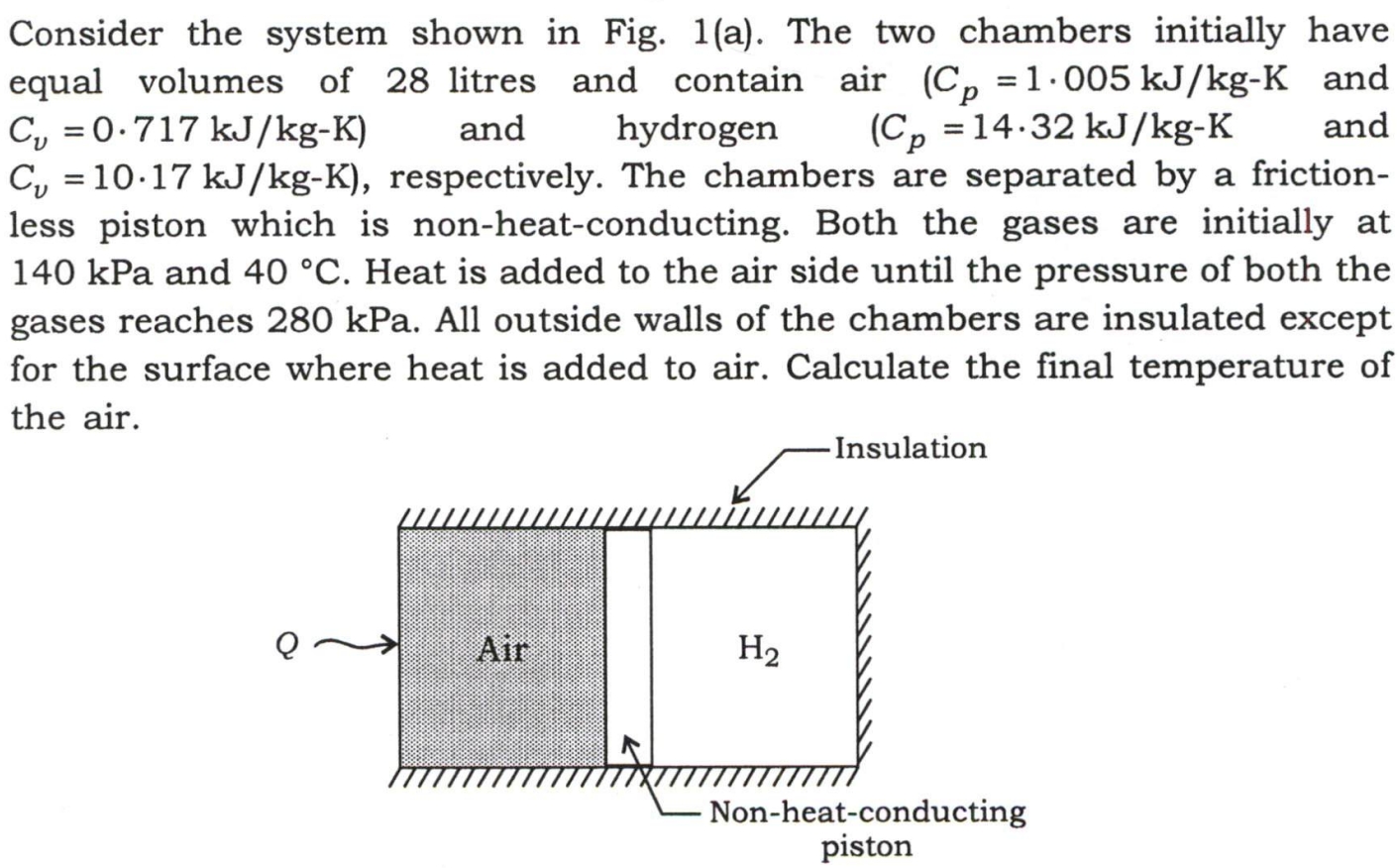
Question: Consider the system shown in Fig. 1 ( a ) . The two chambers initially have equal volumes of 2 8 litres and contain air
Consider the system shown in Fig. a The two chambers initially have
equal volumes of litres and contain air and
: and hydrogen and
: respectively. The chambers are separated by a friction
less piston which is nonheatconducting. Both the gases are initially at
kPa and Heat is added to the air side until the pressure of both the
gases reaches kPa All outside walls of the chambers are insulated except
for the surface where heat is added to air. Calculate the final temperature of
the air.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


