Question: Consider the temperature versus composition diagram for a binary system of substances A and B: Initially, the mixture is at 72 o C and the
Consider the temperature versus composition diagram for a binary system of substances A and B:
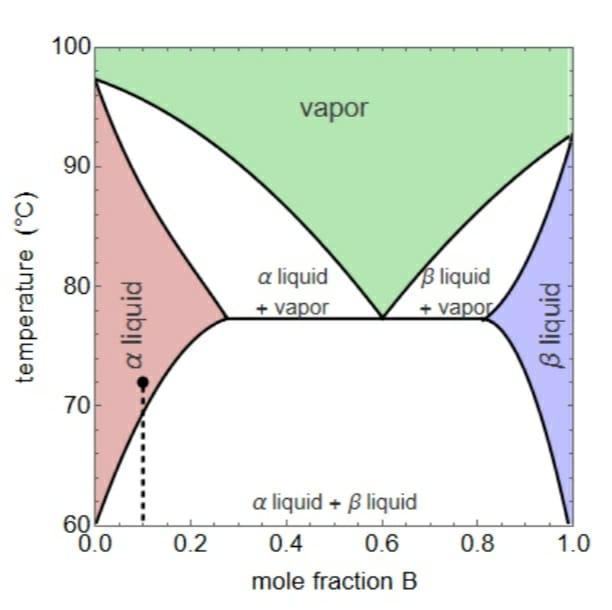
Initially, the mixture is at 72 o C and the molar fraction of B is equal to 0.1.
a) The number of phases and the initial composition of the system.
b) The number of phases and their composition when the molar fraction of B is increased to 0.3.
c) The number of phases and their composition when the molar fraction of B is increased to 0.6.
d) The number of phases and composition of the system when the solution of item c is heated at 76.8 oC.
e) The number of phases and composition of the system when the solution of item c is heated at 80.0 oC
100 vapor 90 a liquid + vapor 80 B liquid + vapor 70 a liquid + B liquid 60 0.0 0.2 0.4 0.6 0.8 1.0 mole fraction B temperature (C) a liquid B liquid
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


