Question: Consider the two different systems, A and B, shown below. If there are 3 particles with a total energy of 12 ), which system would
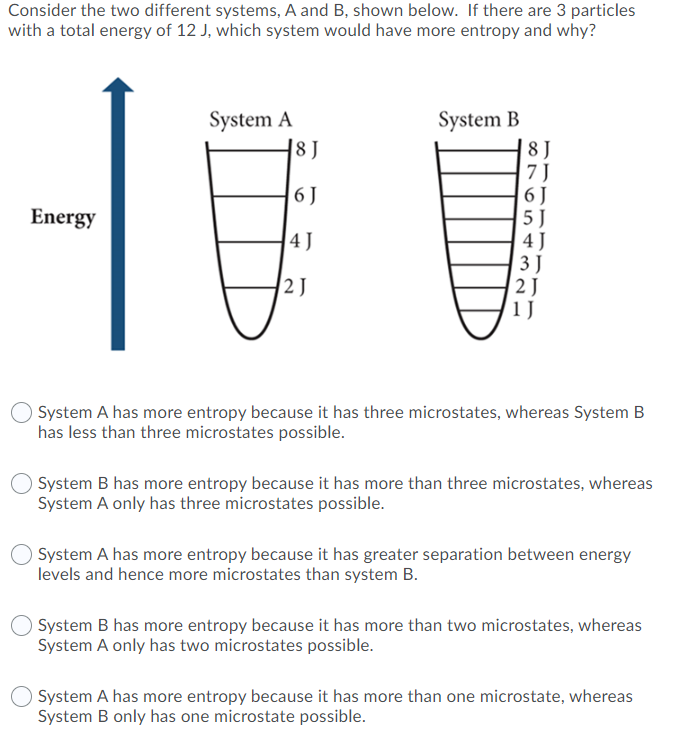
Consider the two different systems, A and B, shown below. If there are 3 particles with a total energy of 12 ), which system would have more entropy and why? System A 18 ) System B | 6J Energy 8 J 7J 6J 5J 4) 3 J ( 2 J 1) 4J 2 J System A has more entropy because it has three microstates, whereas System B has less than three microstates possible. System B has more entropy because it has more than three microstates, whereas System A only has three microstates possible. System A has more entropy because it has greater separation between energy levels and hence more microstates than system B. System B has more entropy because it has more than two microstates, whereas System A only has two microstates possible. System A has more entropy because it has more than one microstate, whereas System B only has one microstate possible
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


