Question: Consider this reaction: 2HI(g)H2(q)+H2(g) At a certain temperature it obeys this rate law. rate=(9.12104s1)[HI] Suppose a vessel contains HI at a concentration of 1.15M. Calculate
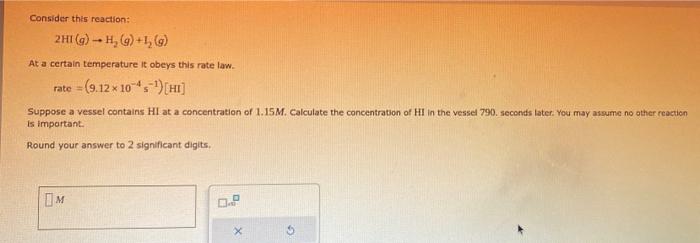
Consider this reaction: 2HI(g)H2(q)+H2(g) At a certain temperature it obeys this rate law. rate=(9.12104s1)[HI] Suppose a vessel contains HI at a concentration of 1.15M. Calculate the concentration of HII in the vessel 790. seconds later. You may asoume no other reactien is impartant. Round your answer to 2 significant digits
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


