Question: Consider two cells, the first with Al and Ag electrodes, and the second with Zn and Ni electrodes, each in appropriate 1.00 M solutions
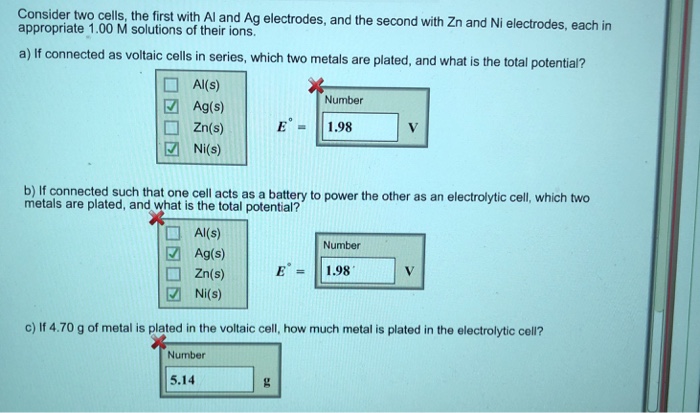
Consider two cells, the first with Al and Ag electrodes, and the second with Zn and Ni electrodes, each in appropriate 1.00 M solutions of their ions. a) If connected as voltaic cells in series, which two metals are plated, and what is the total potential? Al(s) Ag(s) Zn(s) Ni(s) Al(s) Ag(s) Zn(s) Ni(s) E 5.14 Number b) If connected such that one cell acts as a battery to power the other as an electrolytic cell, which two metals are plated, and what is the total potential? g 1.98 Number V 1.98 c) If 4.70 g of metal is plated in the voltaic cell, how much metal is plated in the electrolytic cell? Number V
Step by Step Solution
3.57 Rating (164 Votes )
There are 3 Steps involved in it
If the two cells are connected in series the first cell with Al and Ag electrodes will act as a galvanic cell and generate electricity In this cell the aluminum electrode will be the anode and the sil... View full answer

Get step-by-step solutions from verified subject matter experts


