Question: Calculate the enthalpy of formation of methanol (CH3OH) from its elements, using the heats of combustion below: C (graphite) + 2 H2 (g) +
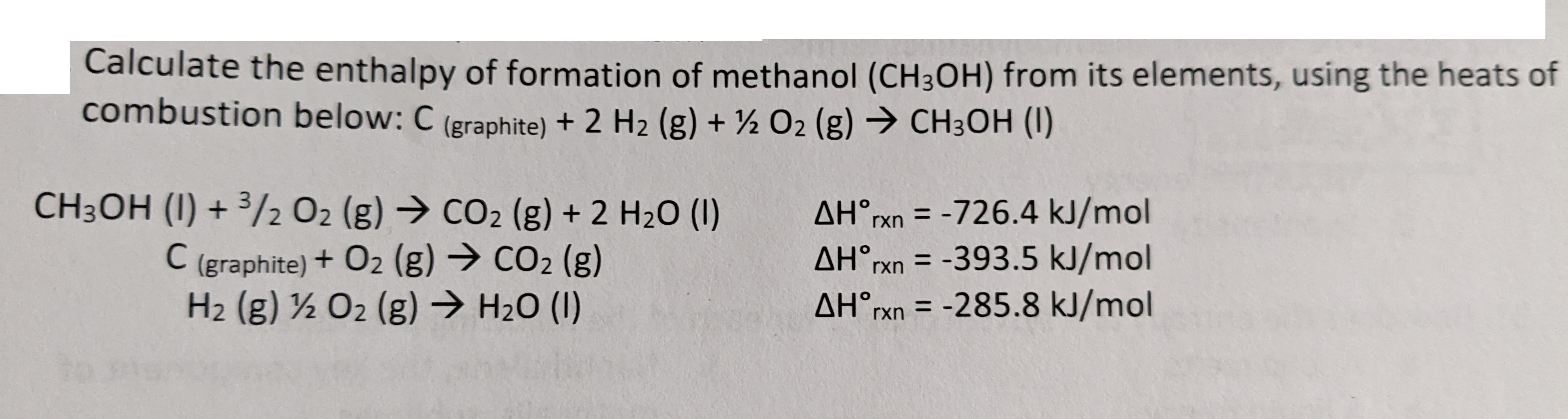
Calculate the enthalpy of formation of methanol (CH3OH) from its elements, using the heats of combustion below: C (graphite) + 2 H2 (g) + O2 (g) CH3OH (1) CH3OH (1) + 3/2 O2 (g) CO2 (g) + 2 H2O (1) C (graphite) + O2 (g) CO2 (g) H2 (g) 12 O2 (g) HO (1) AHrxn=-726.4 kJ/mol AHrxn=-393.5 kJ/mol AHrxn=-285.8 kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


