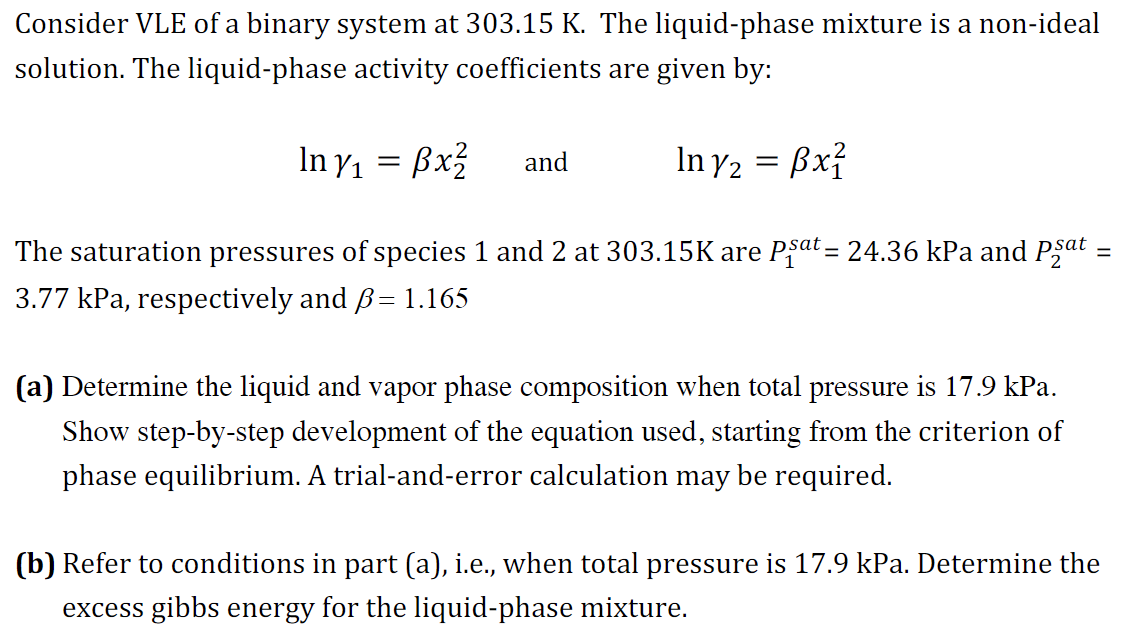
Question: Consider VLE of a binary system at 3 0 3 . 1 5 K . The liquid - phase mixture is a non - ideal
Consider VLE of a binary system at The liquidphase mixture is a nonideal
solution. The liquidphase activity coefficients are given by:
and
The saturation pressures of species and at are kPa and
kPa, respectively and
a Determine the liquid and vapor phase composition when total pressure is kPa.
Show stepbystep development of the equation used, starting from the criterion of
phase equilibrium. A trialanderror calculation may be required.
b Refer to conditions in part a ie when total pressure is kPa. Determine the
excess gibbs energy for the liquidphase mixture.
Please solve it clearly with steps
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


