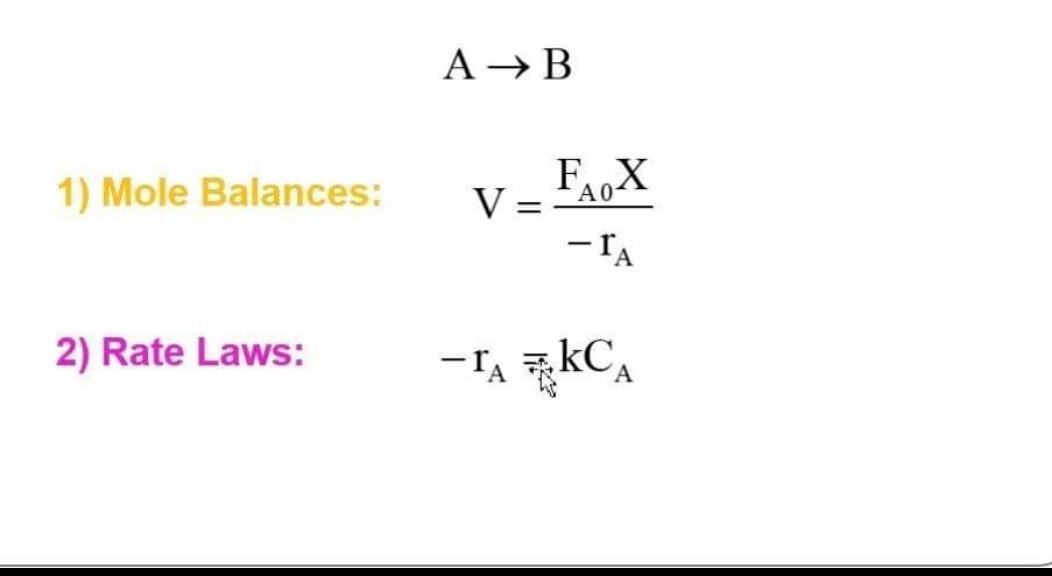
Question: + Considering the reaction is reversible, apply the steps mentioned in the picture and find the conversion equation AB 1) Mole Balances: V=rAFA0X 2) Rate
+
Considering the reaction is reversible, apply the steps mentioned in the picture and find the conversion equation
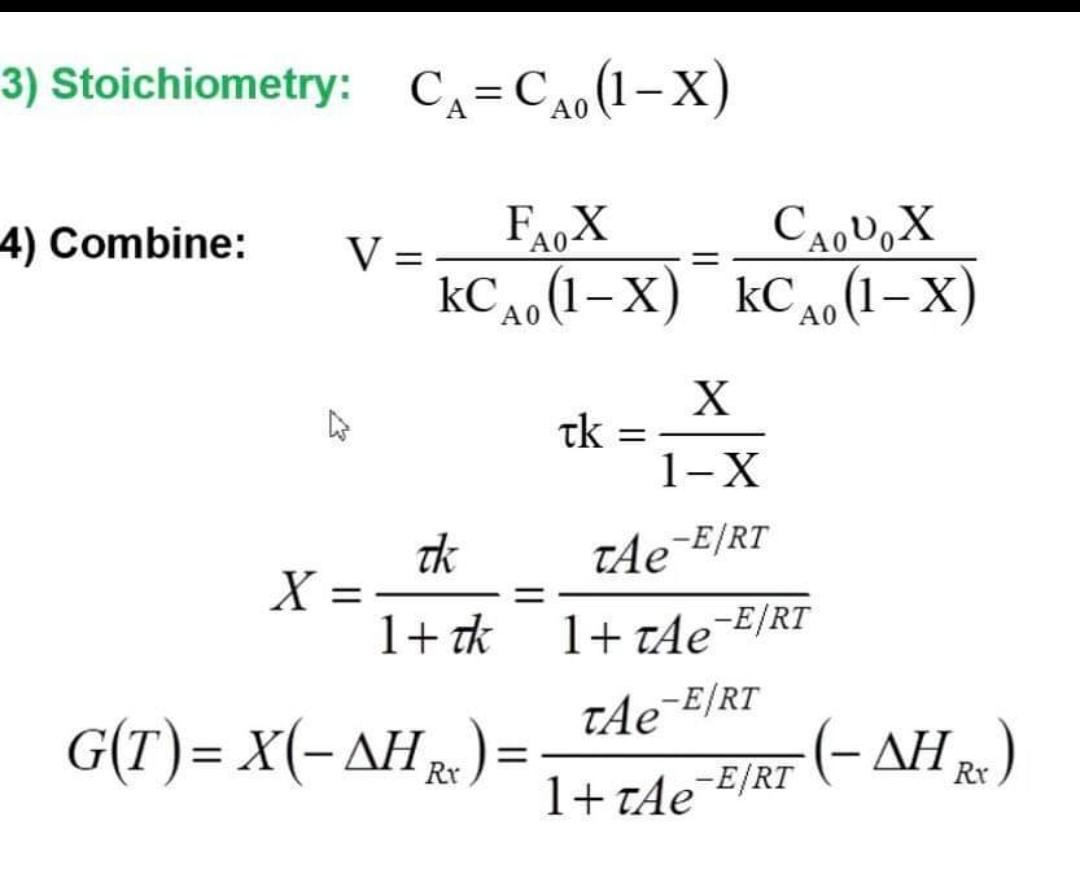
AB 1) Mole Balances: V=rAFA0X 2) Rate Laws: rA=FAkCA 3) Stoichiometry: CA=CA0(1X) 4) Combine: V=kCA0(1X)FA0X=kCA0(1X)CA0v0X k=1XX X=1+kk=1+AeE/RTAeE/RT G(T)=X(HRx)=1+AeE/RTAeE/RT(HRx)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


