Question: Constants I Periodic Table. - Part A Calorimetry is a method used to measure enthalpy, or heat, changes that occur during chemical processes. Two common
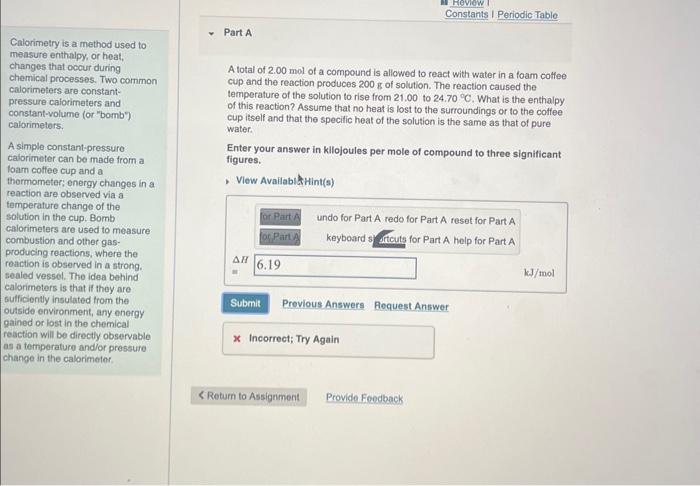
Constants I Periodic Table. - Part A Calorimetry is a method used to measure enthalpy, or heat, changes that occur during chemical processes. Two common calorimeters are constantpressure calorimeters and constant-volume (or "bomb') calorimeters. A total of 2.00mol of a compound is allowed to react with water in a foam coffee cup and the reaction produces 200g of solution. The reaction caused the temperature of the solution to rise from 21.00 to 24.70C. What is the enthalpy of this reaction? Assume that no heat is lost to the surroundings or to the coffee cup itself and that the specific heat of the solution is the same as that of pure water. A simple constant-pressure Enter your answer in kilojoules per mole of compound to three significant calorimeter can be made thermometer; energy changes in a , View Availabilitint(s) reaction are observed via a temperature change of the solution in the cup. Bomb calorimelers are used to measure combustion and other gasproducing reactions, where the reaction is observed in a strong. sealed vessel. The idea behind calorimeters is that if they are sulficiently insulated from the outside environment, any enorgy gained or lost in the chemical reaction will be directly observable as a temperature and/or pressure change in the calorimeter
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


