Question: Constants The following values may be useful when solving this tutorial. Constant Value Ecu 0.337 V E -0.440V R 8.314 J mol-K F 96 485
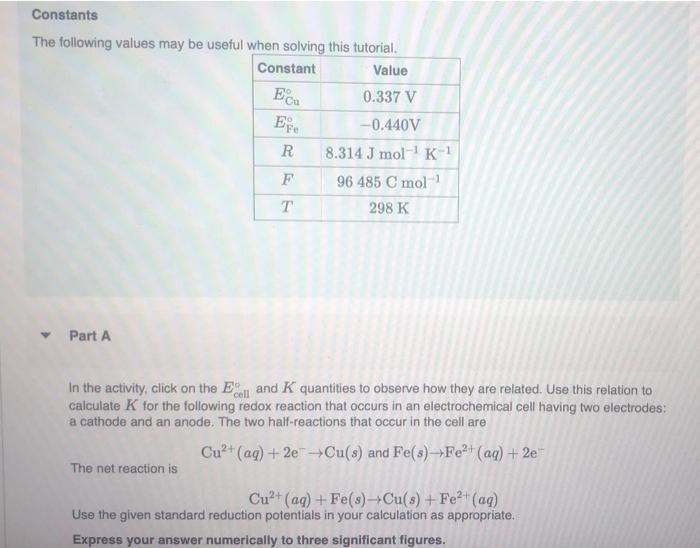
Constants The following values may be useful when solving this tutorial. Constant Value Ecu 0.337 V E -0.440V R 8.314 J mol-K F 96 485 C mol . 298 K Part A In the activity, click on the cell and K quantities to observe how they are related. Use this relation to calculate K for the following redox reaction that occurs in an electrochemical cell having two electrodes: a cathode and an anode. The two half-reactions that occur in the cell are Cu+ (aq) +2e + Cu(s) and Fe(s) Fe2+ (aq) + 2e The net reaction is Cu+ (aq) + Fe(s) Cu(8) + Fe2+ (aq) Use the given standard reduction potentials in your calculation as appropriate. Express your answer numerically to three significant figures. +
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


