Question: Construct the hypothetical binary A-B system which represents the following conditions; Melting temperature of A is 2060C,B is 1800C and AB3 is 1700C, - There

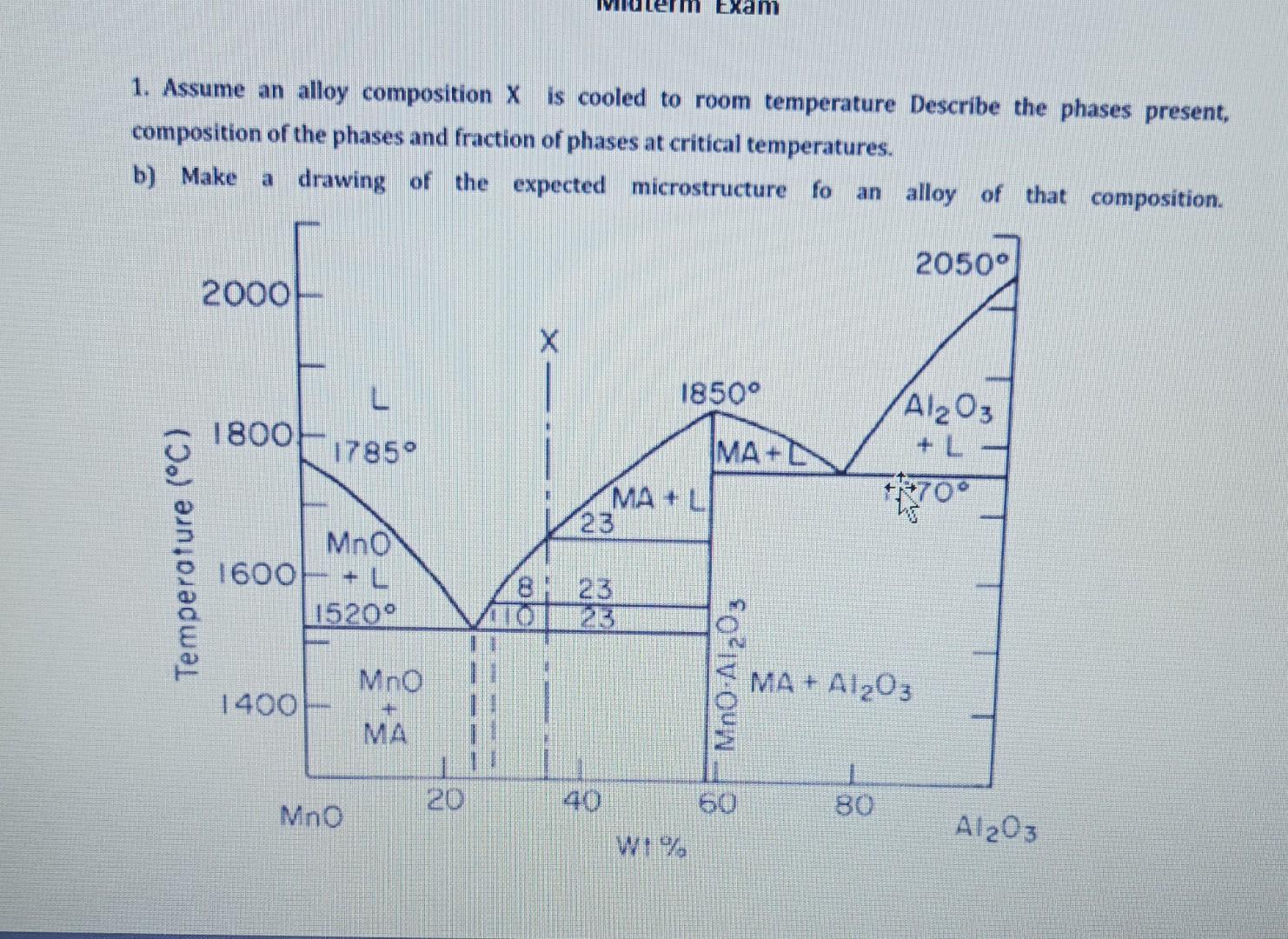
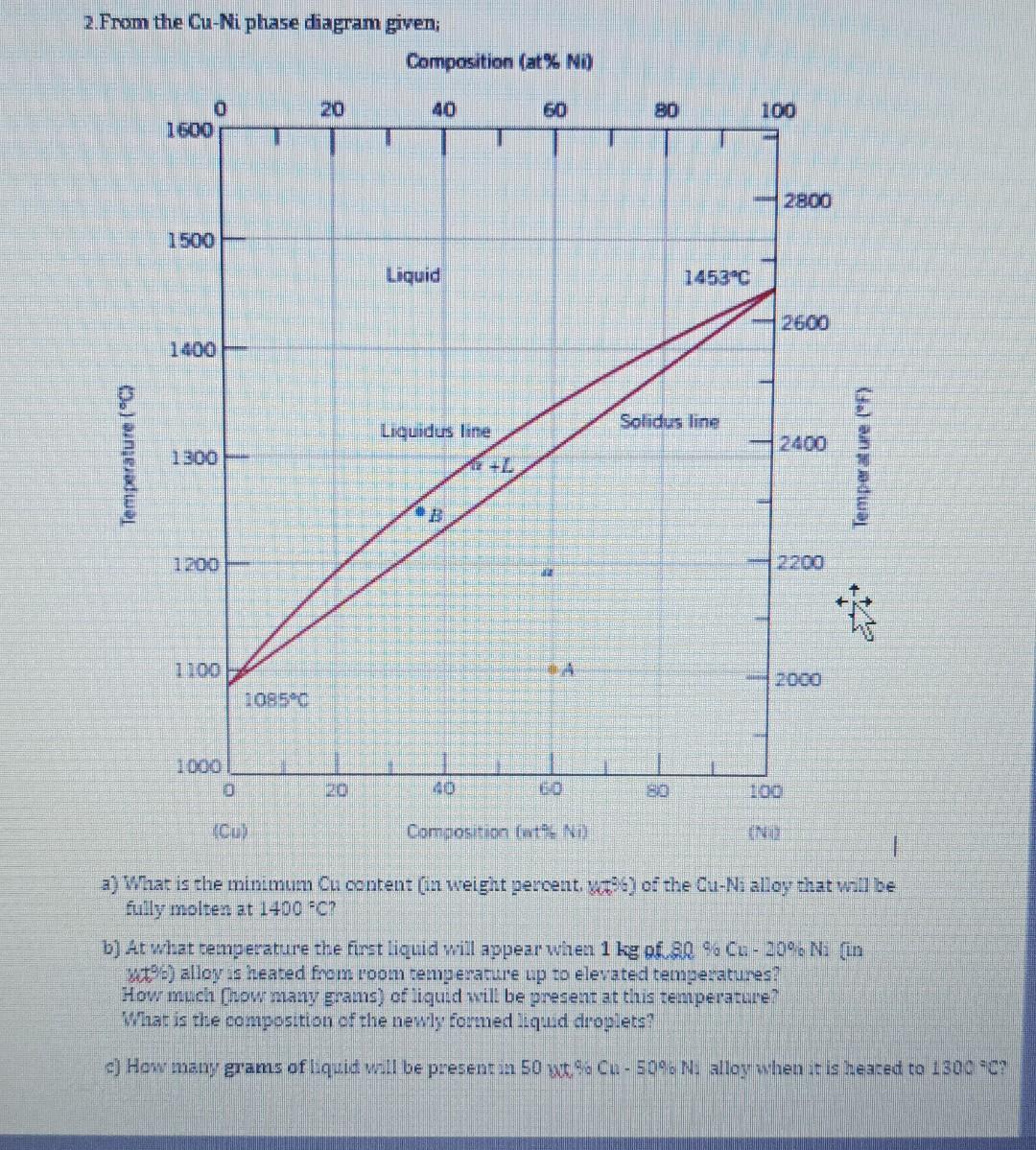
Construct the hypothetical binary A-B system which represents the following conditions; Melting temperature of A is 2060C,B is 1800C and AB3 is 1700C, - There is a peritectic reaction at 1750C; (12mol%B)+L(49mol%B)=(35mol%B) has 3040mol%B and has 2mol%B at room temperature. - There is a eutectic reaction at 1500C; L(57mol%B)=AB3+(36mol%B) - There is a eutectic reaction at 1350C; L (85mol%B)=AB3+B(100mol%B) 1. Assume an alloy composition X is cooled to room temperature Describe the phases present, composition of the phases and fraction of phases at critical temperatures. b) Make a drawing of the expected microstructure fo an alloy of that composition. 2. From the Cu-Mi phase diagram given; a) What is the minimum Cu content (in weight percent, vat of ot the Cu-Ni alloy that will be fully molten at 1400=0 ? b) At what remperature the first liquid will appear when 1kg of 80%6Cu29 o N. [in ut 5 ) alloy is heated from room temperanure up to elevated temperatures? How much (now many grams) of liguid will be gresent at this temperature? What is the composition of the newly formed Lqud droglets? c) How maxy grams of Lhuid will be present an 50 wt 9 Cu. 50%N alloy when it is heared to 1306 se? Construct the hypothetical binary A-B system which represents the following conditions; Melting temperature of A is 2060C,B is 1800C and AB3 is 1700C, - There is a peritectic reaction at 1750C; (12mol%B)+L(49mol%B)=(35mol%B) has 3040mol%B and has 2mol%B at room temperature. - There is a eutectic reaction at 1500C; L(57mol%B)=AB3+(36mol%B) - There is a eutectic reaction at 1350C; L (85mol%B)=AB3+B(100mol%B) 1. Assume an alloy composition X is cooled to room temperature Describe the phases present, composition of the phases and fraction of phases at critical temperatures. b) Make a drawing of the expected microstructure fo an alloy of that composition. 2. From the Cu-Mi phase diagram given; a) What is the minimum Cu content (in weight percent, vat of ot the Cu-Ni alloy that will be fully molten at 1400=0 ? b) At what remperature the first liquid will appear when 1kg of 80%6Cu29 o N. [in ut 5 ) alloy is heated from room temperanure up to elevated temperatures? How much (now many grams) of liguid will be gresent at this temperature? What is the composition of the newly formed Lqud droglets? c) How maxy grams of Lhuid will be present an 50 wt 9 Cu. 50%N alloy when it is heared to 1306 se
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


