Question: Convert all condensed structures to bond line structures. Convert all bond line structures to condensed structures. Indicate all non-bonding electrons (e.g., lone pair or unpaired
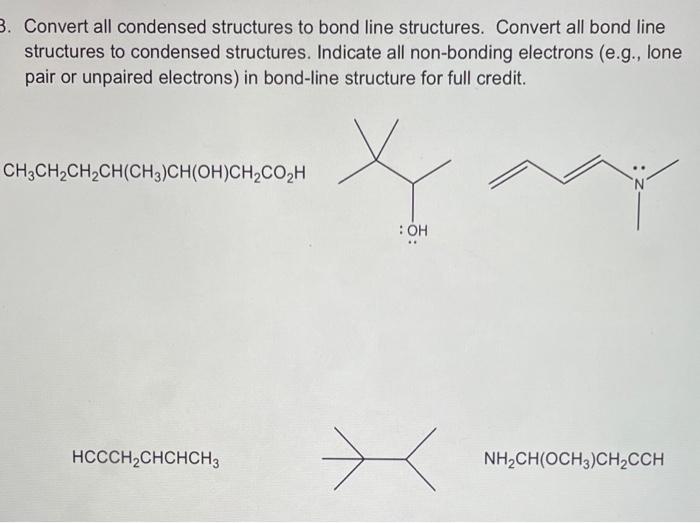
Convert all condensed structures to bond line structures. Convert all bond line structures to condensed structures. Indicate all non-bonding electrons (e.g., lone pair or unpaired electrons) in bond-line structure for full credit. CH3CH2CH2CH(CH3)CH(OH)CH2CO2H HCCCH2CHCHCH3 NH2CH(OCH3)CH2CCH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


