Question: convert to Cm Mass Diameter Length Volume Density (g) (cm) (cm) (cm (g/em3) Metal # 1 A 801039 | 2. 103 0m 3. 2 2
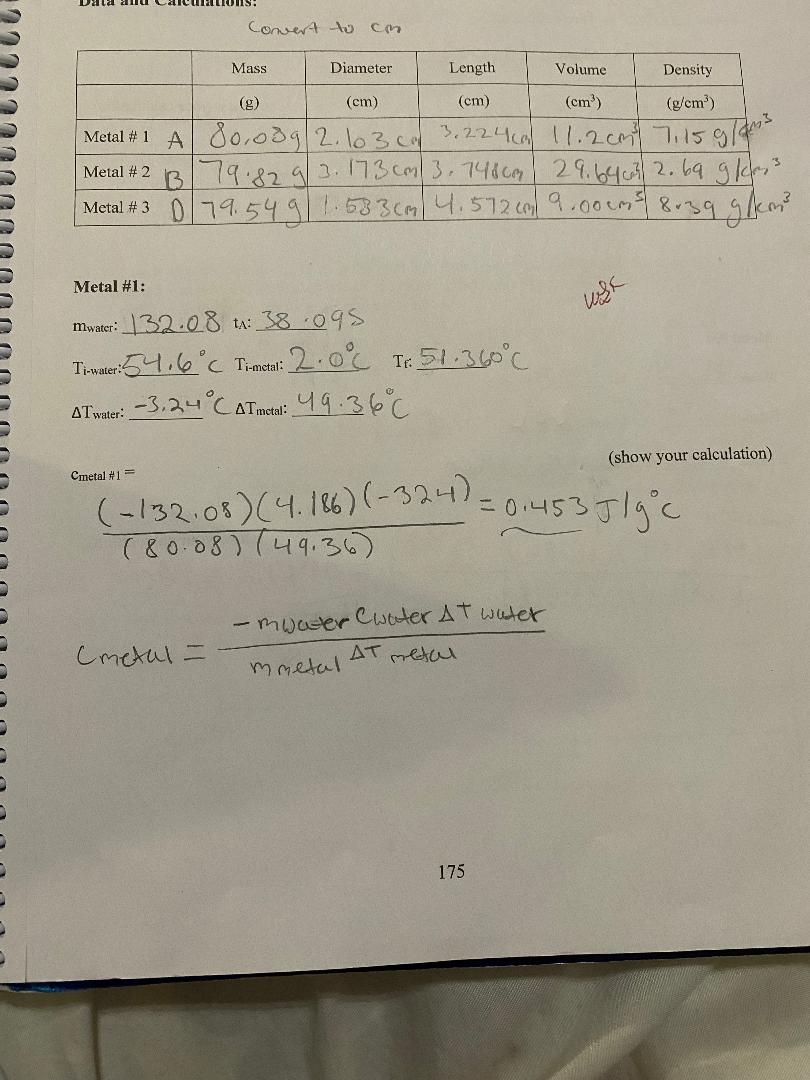
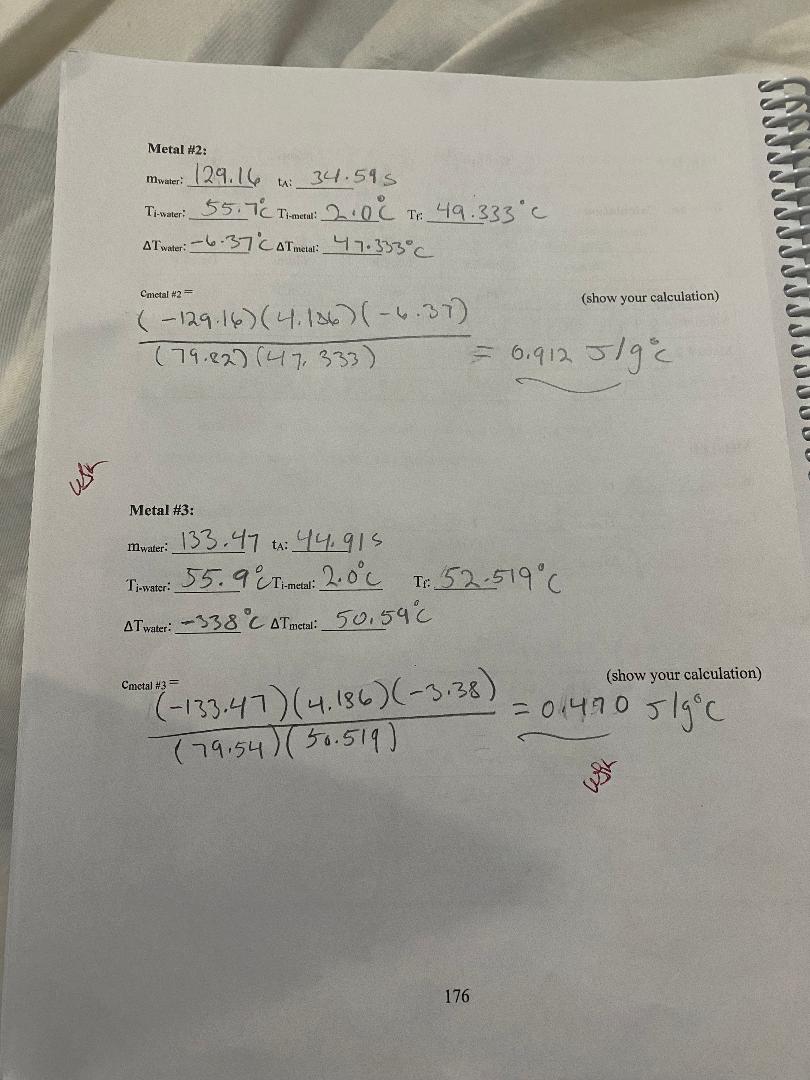
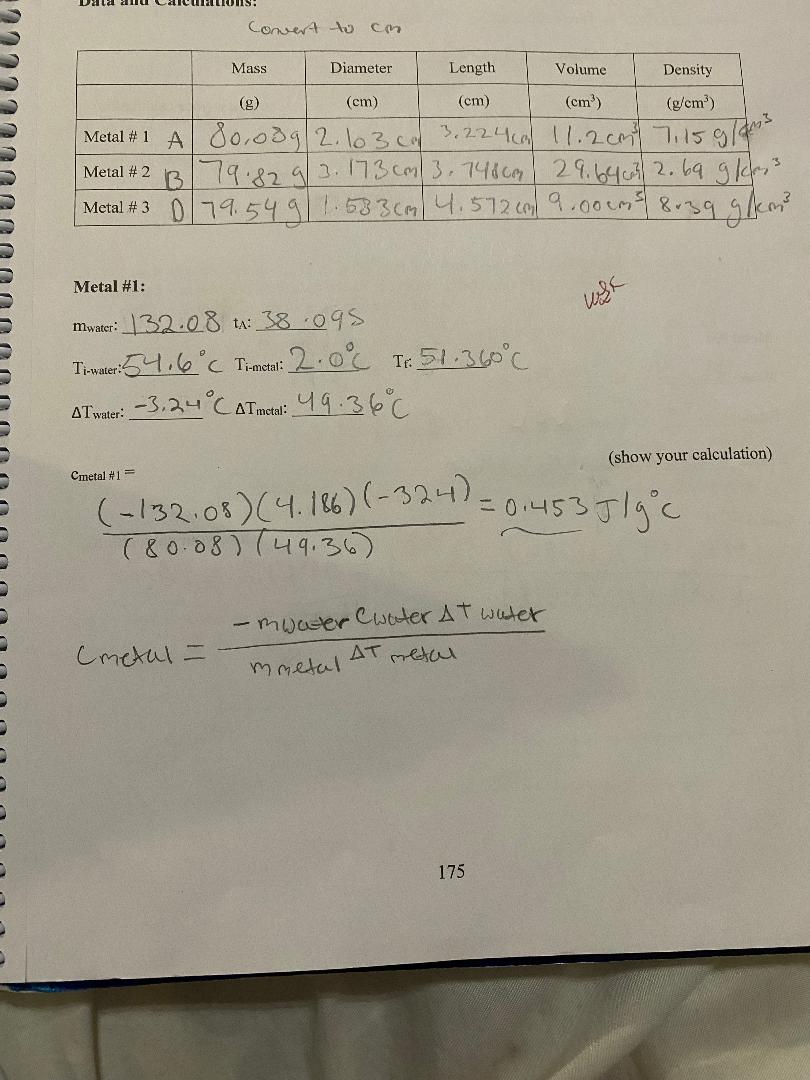
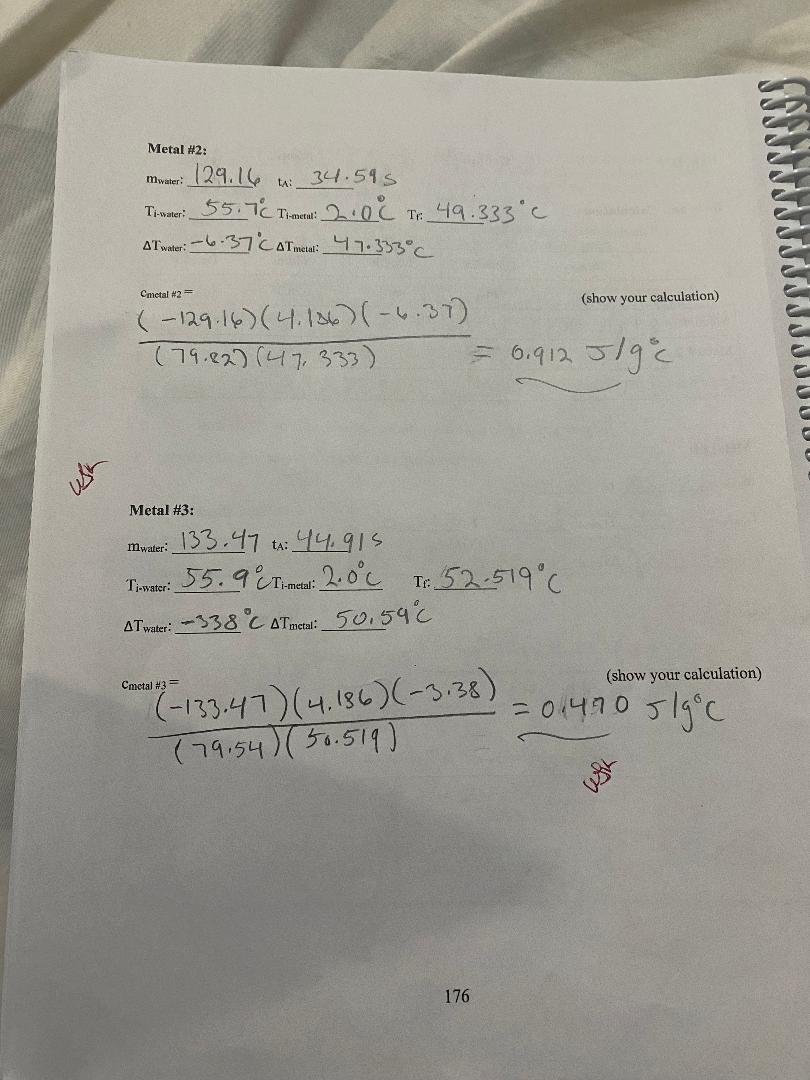
![839 9 /kem] Metal #1: mwater: 3208 +: 38 095 TimWater: 54.6](https://s3.amazonaws.com/si.experts.images/answers/2024/06/6677a0c168fcf_9696677a0c14d612.jpg)
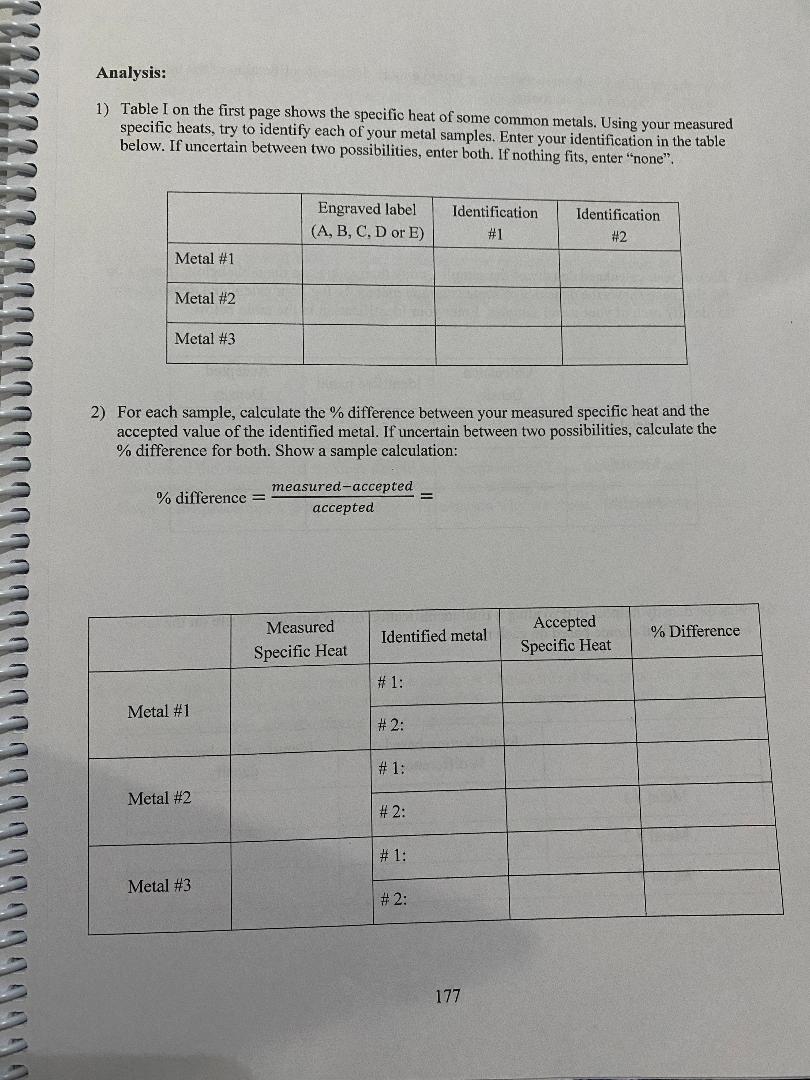
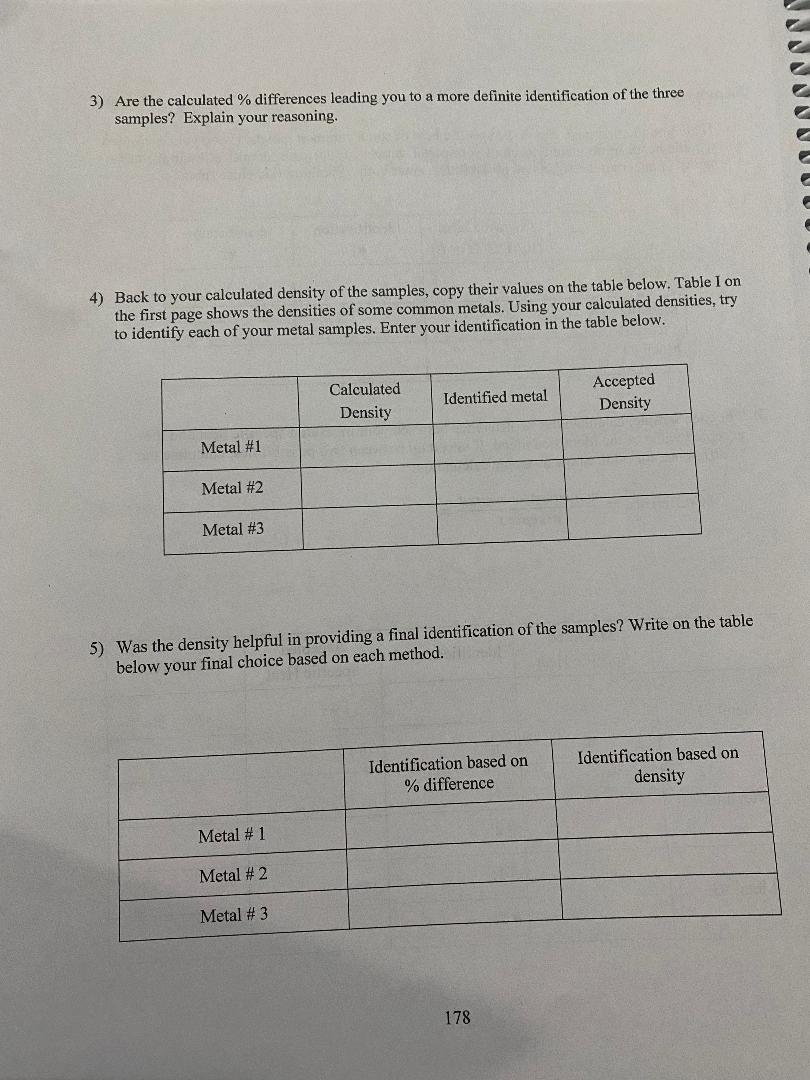
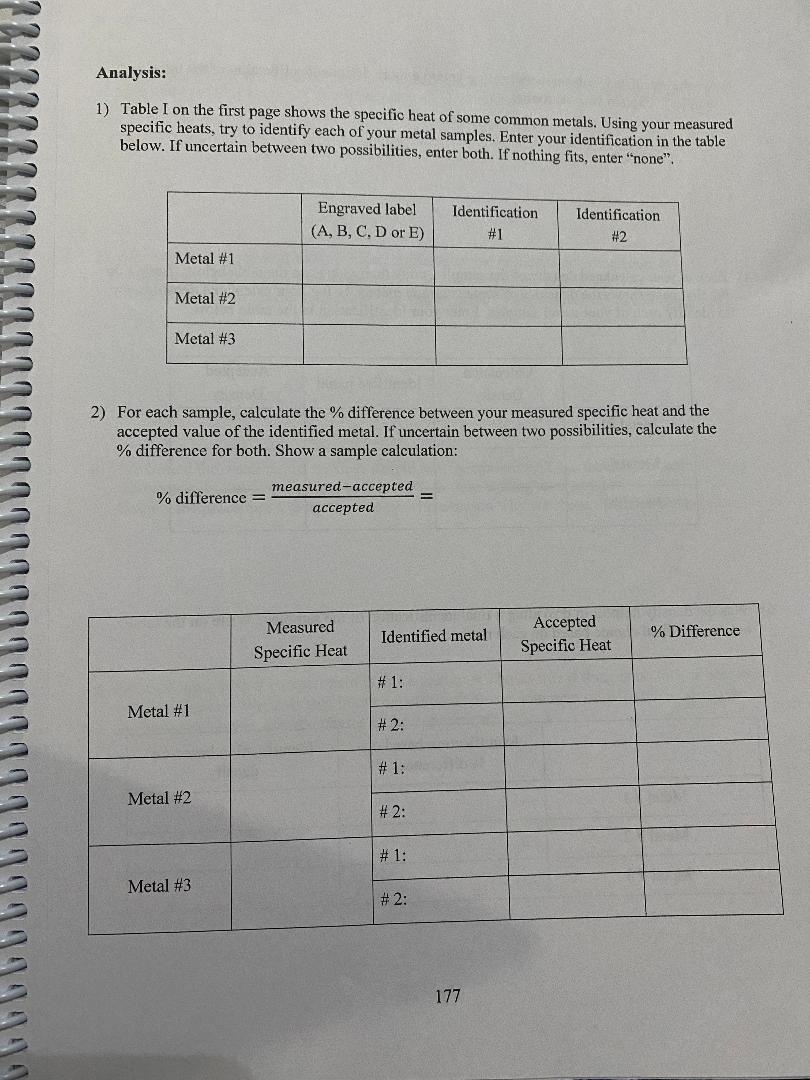
convert to Cm Mass Diameter Length Volume Density (g) (cm) (cm) (cm (g/em3) Metal # 1 A 801039 | 2. 103 0m 3. 2 2 4 ca) 11.2 cm) 7:15 9/ ms Metal # 2 B 179.82 9/ 2. 173 com/ 3. 7480m 2 9. 64 603/ 2. 69 9 /cr, 3 Metal # 3 D 79. 54 9 1 58 3 (m | 4 . 572 (m/ 9. 00 (ms) 839 9 /kem] Metal #1: mwater: 3208 +: 38 095 TimWater: 54.6 C Timetal: 2.0 6 TH: 51. 360 C AT water: - 3. 24 CATmetal: 4 9. 36 C (show your calculation) Cmetal #1= ( -132 08 ) ( 4. 186) (- 324) = 0 1 45 3 J / g c ( 8 0 . 08 ) ( 49. 36 ) - mwater Cwater AT water Cmetal = mmetal AT metal 175\f3) Are the calculated % differences leading you to a more definite identification of the three samples? Explain your reasoning. 4) Back to your calculated density of the samples, copy their values on the table below. Table I on the first page shows the densities of some common metals. Using your calculated densities, try to identify each of your metal samples. Enter your identification in the table below. Calculated Accepted Identified metal Density Density Metal #1 Metal #2 Metal #3 5) Was the density helpful in providing a final identification of the samples? Write on the table below your final choice based on each method. Identification based on Identification based on % difference density Metal # 1 Metal # 2 Metal # 3 178Analysis: 1) Table I on the first page shows the specific heat of some common metals. Using your measured specific heats, try to identify each of your metal samples. Enter your identification in the table below. If uncertain between two possibilities, enter both. If nothing fits, enter "none". Engraved label Identification Identification (A, B, C. D or E) #1 #2 Metal #1 Metal #2 Metal #3 2) For each sample, calculate the % difference between your measured specific heat and the accepted value of the identified metal. If uncertain between two possibilities, calculate the % difference for both. Show a sample calculation: % difference = - measured-accepted accepted Measured Accepted Identified metal % Difference Specific Heat Specific Heat # 1: Metal #1 # 2: # 1: Metal #2 # 2: # 1: Metal #3 # 2: 177
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


