Question: convert to Pa and solve. How many collisions does a single Ar atom, with collisional cross-section =0.36nm2, make in 1.0s when the temperature is 25C
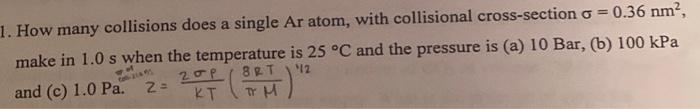
How many collisions does a single Ar atom, with collisional cross-section =0.36nm2, make in 1.0s when the temperature is 25C and the pressure is (a) 10Bar, (b) 100kPa and (c) 1.0Paz=KT2P(H8RT)1/2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


