Question: copper Copper is being diffused into one surface of a part initially composed of a 99.85 wt% aluminum/0.15 wt% copper alloy. The part is
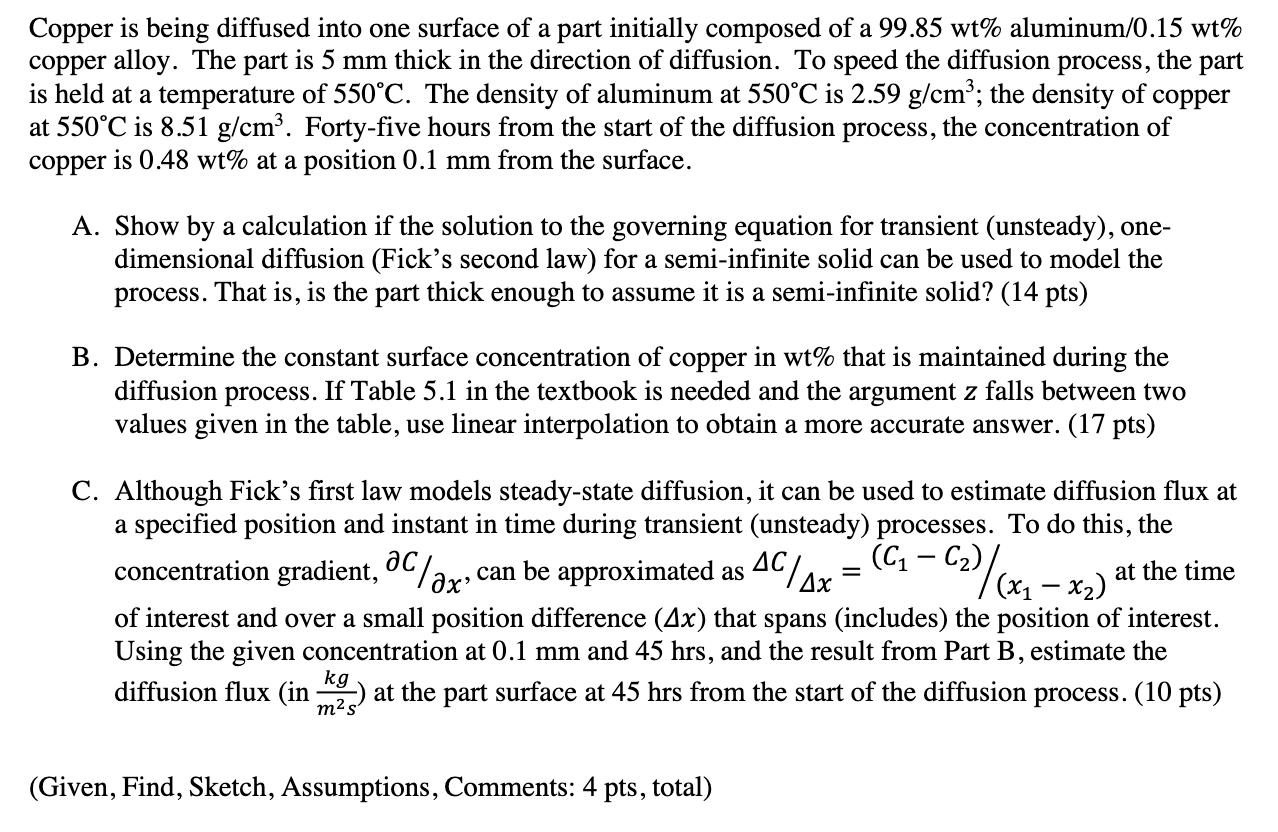
copper Copper is being diffused into one surface of a part initially composed of a 99.85 wt% aluminum/0.15 wt% copper alloy. The part is 5 mm thick in the direction of diffusion. To speed the diffusion process, the part is held at a temperature of 550C. The density of aluminum at 550C is 2.59 g/cm; the density of at 550C is 8.51 g/cm. Forty-five hours from the start of the diffusion process, the concentration of is 0.48 wt% at a position 0.1 mm from the surface. copper A. Show by a calculation if the solution to the governing equation for transient (unsteady), one- dimensional diffusion (Fick's second law) for a semi-infinite solid can be used to model the process. That is, is the part thick enough to assume it is a semi-infinite solid? (14 pts) B. Determine the constant surface concentration of copper in wt% that is maintained during the diffusion process. If Table 5.1 in the textbook is needed and the argument z falls between two values given in the table, use linear interpolation to obtain a more accurate answer. (17 pts) C. Although Fick's first law models steady-state diffusion, it can be used to estimate diffusion flux at a specified position and instant in time during transient (unsteady) processes. To do this, the concentration gradient, C/x" (C1 can be approximated as AC/Ax = - C2)/(x1 - x2) at the time of interest and over a small position difference (4x) that spans (includes) the position of interest. Using the given concentration at 0.1 mm and 45 hrs, and the result from Part B, estimate the 2) at the part surface at 45 hrs from the start of the diffusion process. (10 pts) diffusion flux (in kg m s (Given, Find, Sketch, Assumptions, Comments: 4 pts, total)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


