Question: Correction: The equation is 1/-rA = 0.0985 + 0.3615X + 1.1911X^2 The catalytic reaction A -> 4R is run in a packed bed reactor with
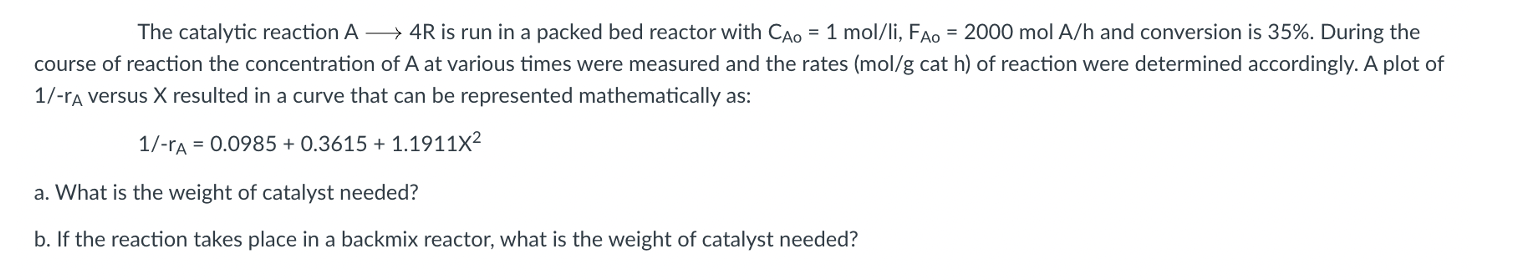
Correction: The equation is 1/-rA = 0.0985 + 0.3615X + 1.1911X^2
The catalytic reaction A -> 4R is run in a packed bed reactor with Cao = 1 mol/li, Fao = 2000 mol A/h and conversion is 35%. During the course of reaction the concentration of A at various times were measured and the rates (mol/g cat h) of reaction were determined accordingly. A plot of 1/-ra versus X resulted in a curve that can be represented mathematically as: 1/-rA = 0.0985 + 0.3615 + 1.191112 a. What is the weight of catalyst needed? b. If the reaction takes place in a backmix reactor, what is the weight of catalyst needed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


