Question: CORROSION ENGINEERING. PLEASE ANSWER QUESTION COMPLETELY WITH STEPS. I WILL LEAVE A GOOD RATING VERY VERY QUICKLY. I PROMISE! Problem 3 Evan's Diagram (Note: difficult
CORROSION ENGINEERING. PLEASE ANSWER QUESTION COMPLETELY WITH STEPS. I WILL LEAVE A GOOD RATING VERY VERY QUICKLY. I PROMISE!
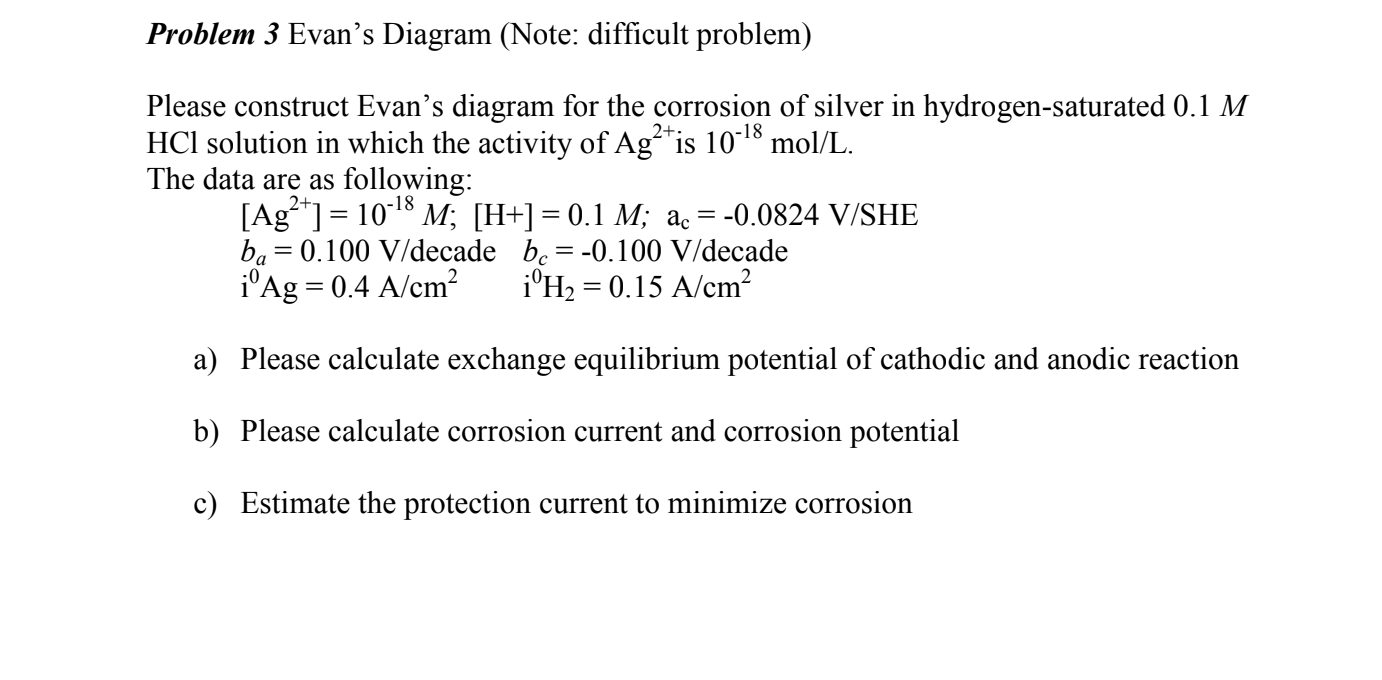
Problem 3 Evan's Diagram (Note: difficult problem) Please construct Evan's diagram for the corrosion of silver in hydrogen-saturated 0.1M HCl solution in which the activity of Ag2+ is 1018mol/L. The data are as following: [Ag2+]=1018M;[H+]=0.1M;ac=0.0824V/SHEba=0.100V/decadebc=0.100V/decadei0Ag=0.4A/cm2i0H2=0.15A/cm2 a) Please calculate exchange equilibrium potential of cathodic and anodic reaction b) Please calculate corrosion current and corrosion potential c) Estimate the protection current to minimize corrosion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


