Question: could solve the problem please and thanks ..I want to make sure of my answers. Q1: 1000 moles of 60% methanol and 40% water mixture
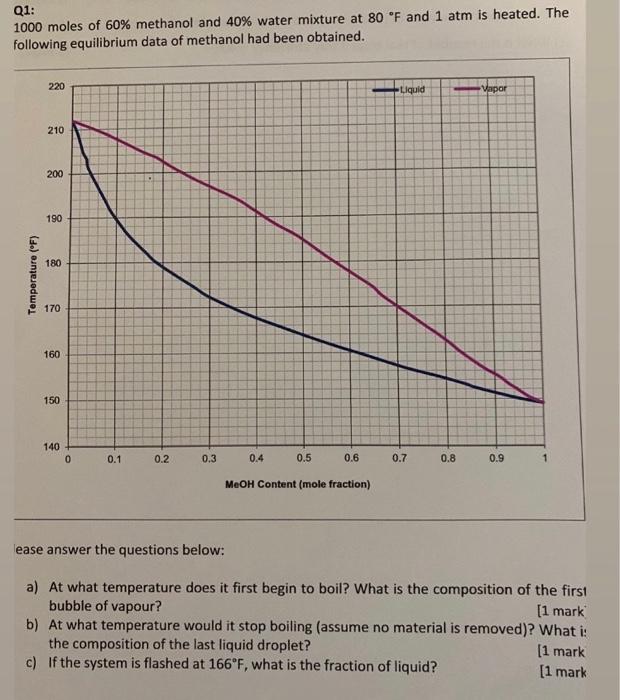
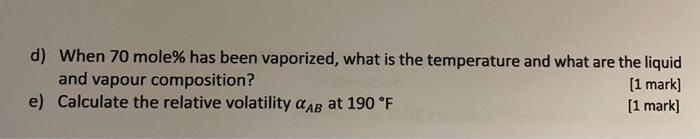
Q1: 1000 moles of 60% methanol and 40% water mixture at 80 F and 1 atm is heated. The following equilibrium data of methanol had been obtained. 220 Liquid Vapor 210 200 190 180 Temperature (F) 170 160 150 140 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 MeOH Content (mole fraction) lease answer the questions below: a) At what temperature does it first begin to boil? What is the composition of the first bubble of vapour? [1 mark b) At what temperature would it stop boiling (assume no material is removed)? What is the composition of the last liquid droplet? [1 mark c) If the system is flashed at 166F, what is the fraction of liquid? [1 mark d) When 70 mole% has been vaporized, what is the temperature and what are the liquid and vapour composition? [1 mark] e) Calculate the relative volatility AB at 190 F [1 mark]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


