Question: could someone help me with the calculations and correct the data pls? Data and Observations: Data Table 1: Effect of Concentration (Stock KIO3 concentration is
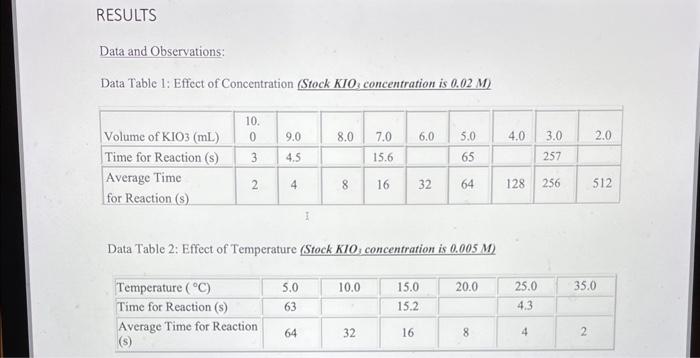
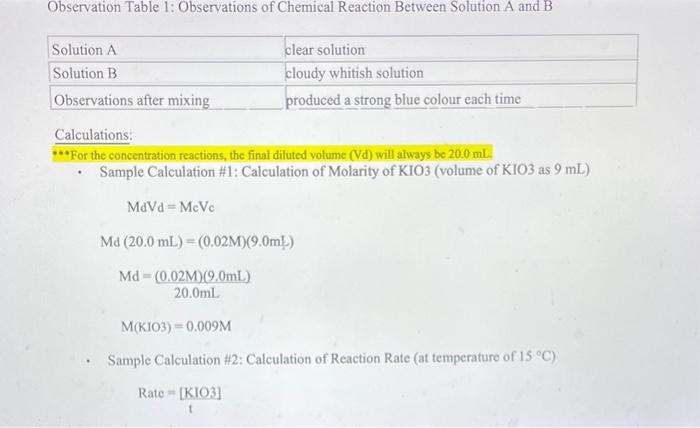
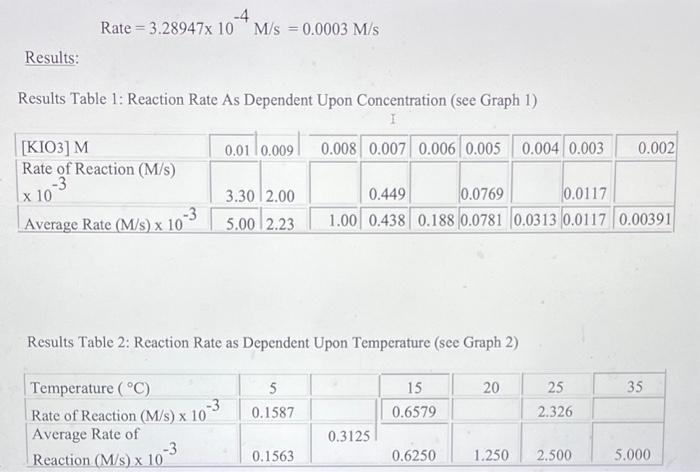
Data and Observations: Data Table 1: Effect of Concentration (Stock KIO3 concentration is 0.02M ) Data Table 2: Effect of Temperature (Stock KIO3 concentration is 0.005M) Calculations: **"For the concentration reactions, the final diluted volume (Vd) will always be 20.0mL. - Sample Calculation \#1: Calculation of Molarity of KIO3 (volume of KIO3 as 9mL ) MdVd=McVcMd(20.0mL)=(0.02M)(9.0mL)Md=20.0mL(0.02M)(9.0mL)M(KIO)=0.009M - Sample Calculation \#2: Calculation of Reaction Rate (at temperature of 15C ) Rate=t[KIO3] Rate=3.28947104M/s=0.0003M/s Results: Results Table 1: Reaction Rate As Dependent Upon Concentration (see Graph 1) Results Table 2: Reaction Rate as Dependent Upon Temperature (see Graph 2)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


