Question: could someone help sovle this with steps fast plzzzz 10 11 12 13 14 15 17 18 2. You are part of a team of
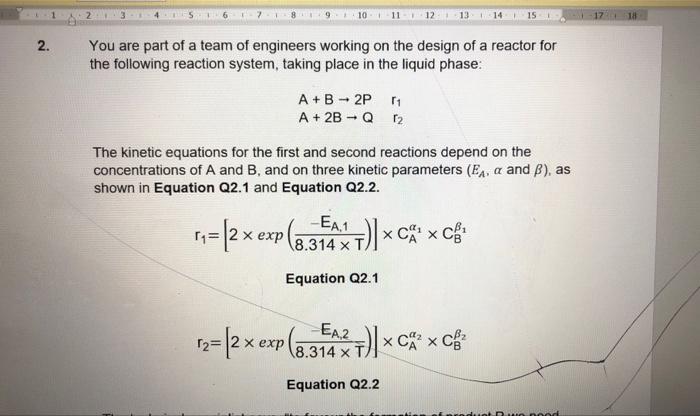

10 11 12 13 14 15 17 18 2. You are part of a team of engineers working on the design of a reactor for the following reaction system, taking place in the liquid phase: A+B - 2P 11 A + 2B - Q2 The kinetic equations for the first and second reactions depend on the concentrations of A and B, and on three kinetic parameters (Ex, a and B), as shown in Equation Q2.1 and Equation Q2.2. 11=(2x exp (@ (8.314T) x C x C# Equation Q2.1 1 = 2x exp(6.349% +)*cxcf = T) Equation Q2.2 The technical specialist says: "to favour the formation of product P we need to use a tubular reactor, with dosing of B, at the lowest temperature possible". (a) Using the information given, compare the values of the kinetic parameters for reactions 1 and 2, and show qualitatively how the technical specialist reached that conclusion (10 marks) (b) In the continuous stirred tank reactor used as a pilot plant, we introduce 5 mols of A and 20 mol s'of B. At the outlet, the ratio of selectivity of Pover Q, Sp/q, is equivalent to 2, and the molar flow rate of Pis 3 mol s Calculate the conversion of the reactants and the total molar flow rate at the outlet of the reactor (10 marks) (c) Describe three chemical properties for this reaction and explain the main aspects of design related to them
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


