Question: Could someone please explain the answer explanations for this question? 39. Samples of NaF(s) and NH4Cl(s) are dissolved in separate beakers that each contain 100mL
Could someone please explain the answer explanations for this question?
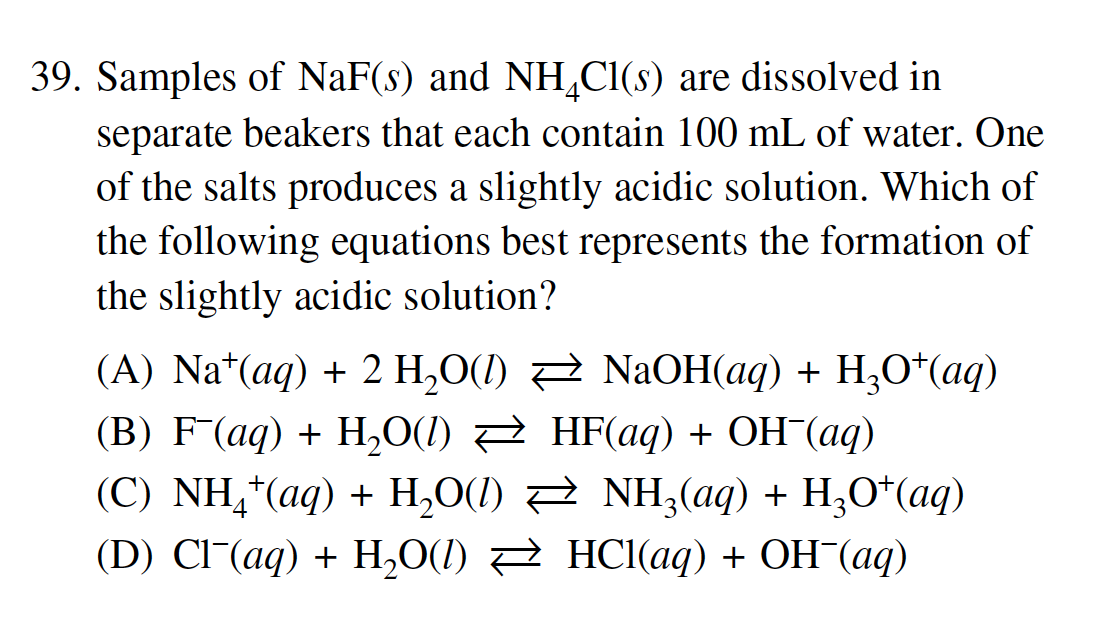
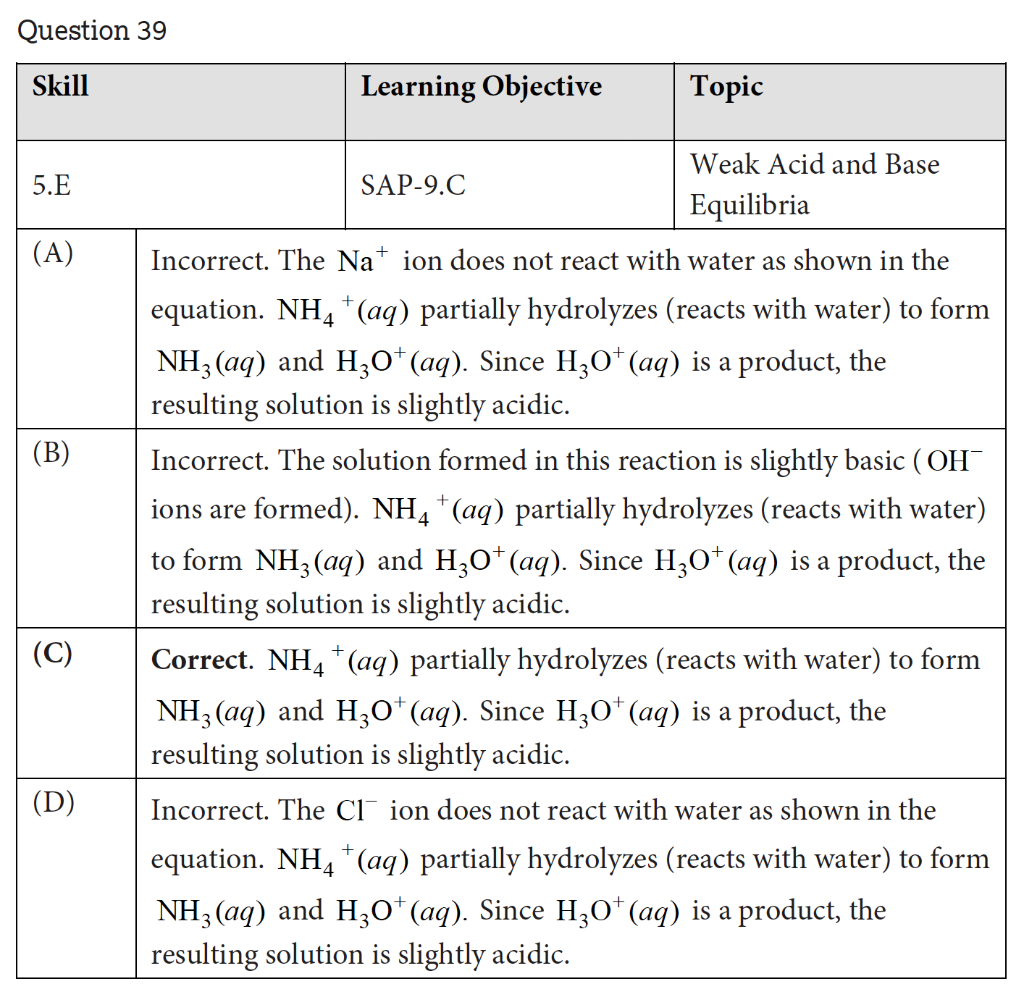
39. Samples of NaF(s) and NH4Cl(s) are dissolved in separate beakers that each contain 100mL of water. One of the salts produces a slightly acidic solution. Which of the following equations best represents the formation of the slightly acidic solution? (A) Na+(aq)+2H2O(l)NaOH(aq)+H3O+(aq) (B) F(aq)+H2O(l)HF(aq)+OH(aq) (C) NH4+(aq)+H2O(l)NH3(aq)+H3O+(aq) (D) Cl(aq)+H2O(l)HCl(aq)+OH(aq) Question 39
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


