Question: Could someone please help me with these two problems? Thanks in advance 2. Consider a solution that contains Cu2+ and Cl. Given the logK for
Could someone please help me with these two problems? Thanks in advance
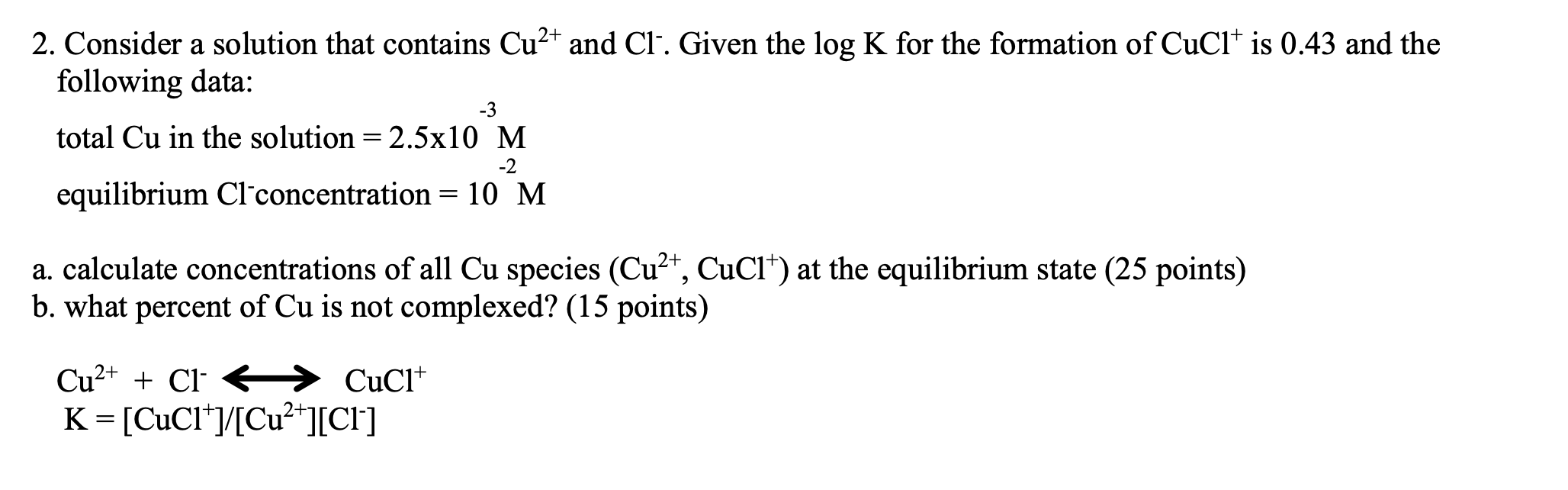
2. Consider a solution that contains Cu2+ and Cl. Given the logK for the formation of CuCl+is 0.43 and the following data: total Cu in the solution =2.5103M equilibrium Clconcentration =102M a. calculate concentrations of all Cu species (Cu2+,CuCl+)at the equilibrium state ( 25 points) b. what percent of Cu is not complexed? (15 points) Cu2++ClK=[CuCl+]/[Cu2+][Cl]CuCl+
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


