Question: could u plz provide we handwritten solution not typed could u plz do it more detailed then because for part a i get 1.88v instead
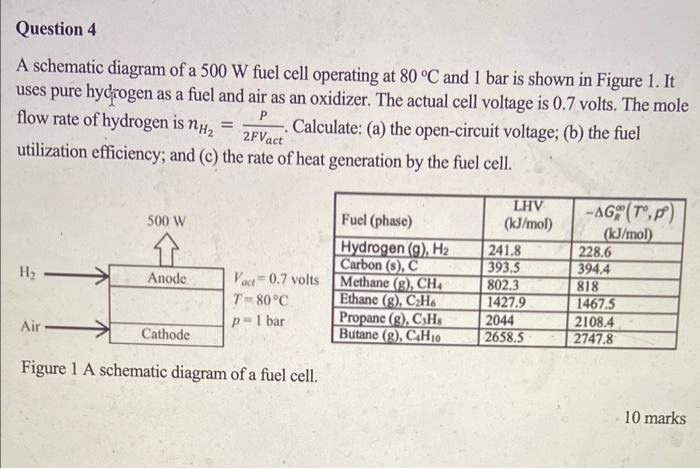
A schematic diagram of a 500W fuel cell operating at 80C and 1 bar is shown in Figure 1 . It uses pure hydrogen as a fuel and air as an oxidizer. The actual cell voltage is 0.7 volts. The mole flow rate of hydrogen is nH2=2FVactP. Calculate: (a) the open-circuit voltage; (b) the fuel utilization efficiency; and (c) the rate of heat generation by the fuel cell. Figure 1 A schematic diagram of a fuel cell. 10 marks
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


