Question: could you also explain how you did this please? 1) What is the molarity of nitrate (NO3-) in a solution that is 5.42 w/w %
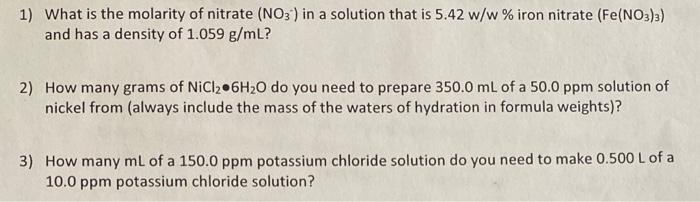
1) What is the molarity of nitrate (NO3-) in a solution that is 5.42 w/w % iron nitrate (Fe(NO3)3) and has a density of 1.059 g/mL? 2) How many grams of NiCl 6H20 do you need to prepare 350.0 mL of a 50.0 ppm solution of nickel from (always include the mass of the waters of hydration in formula weights)? 3) How many mL of a 150.0 ppm potassium chloride solution do you need to make 0.500 L of a 10.0 ppm potassium chloride solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


