Question: could you help me answer 1-3 pls Theoretical yield just needs to be calculated. The experiment has not been done yet. Fease note of reacrion
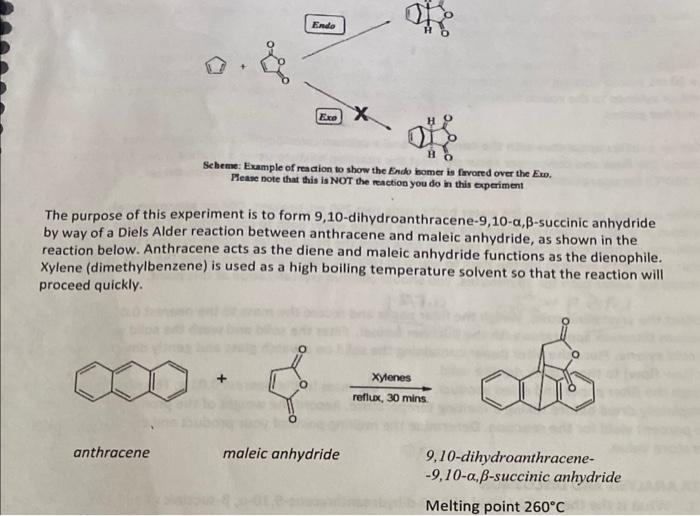


Fease note of reacrion to show the Endo bomer is favored over the Eim. The purpose of this experiment is to form 9,10-dihydroanthracene-9,10- ,-succinic anhydride by way of a Diels Alder reaction between anthracene and maleic anhydride, as shown in the reaction below. Anthracene acts as the diene and maleic anhydride functions as the dienophile. Xylene (dimethylbenzene) is used as a high boiling temperature solvent so that the reaction will proceed quickly. reflux,30minsxytenes anthracene maleicanhydride9,10-dihydroanthracene-9,10,-succinicanhydride Melting point 260C 1. What is the theoretical yield of 9,10-dihydroanthracene-9,10- ,-succinic anhydride in your synthesis? 2. What is the actual yield? 3. What is the percentage yield? Show all your calculations PROCEDURE Wear goggles. If possible, protect your arms and hands by wearing a long-sleeve lab coat and gloves. Conduct this reaction in a fume hood. Weigh out 0.60g of anthracene and 0.30g of maleic anhydride and transfer the reagents into a 10mL round bottom flask containing a spin vane. Record both masses to the nearest 0.01g. Add 6 mL of xylenes to the round bottom flask. CAUTION: Xylene is flammable. Keep away from open flames and hot plates. Set up a reflux apparatus with the condenser and a calcium chloride-filled drying tube, making sure to clamp the flask and condenser securely. Heat the reaction mixture using a heating mantle to reflux ( 180C ) for approximately 30 minutes. Monitor the temperature using a thermometer. While waiting, prepare two ice water baths using two 250mL beakers. Obtain approximately 10mL of xylenes in a 100mL beaker. Place the beaker in the ice water bath to cool. After 30 minutes of refluxing is complete, remove the reaction flask from the heat and let it come to room temperature. Wait at least 15 minutes for the flask to warm to room temperature. Then, place the flask in the second ice water bath for 10 minutes. You should observe crystallization at this point. Collect the crystalline solid. Weigh the filter paper and record the mass to the nearest 0.01g. Set up a vacuum filtration apparatus with the Bchner funnel. Filter the solid and wash the solid with 510mL of cold xylene. Place the filter paper containing the solid on a watch glass and gently direct a stream of air (low flow) to thoroughly dry the solid. Weigh the filter paper and dried product. Record the mass to the nearest 0.01g. The sample should be completely dried before taking a melting temperature. Record the weight of the product and calculate the percent yield for the reaction. Determine the melting point of your product and compare it to the
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


