Question: The flask shown here contains 0.130 g of acid and a few drops of phenolphthalein indicator dissolved in water. The buret contains 0.160 M
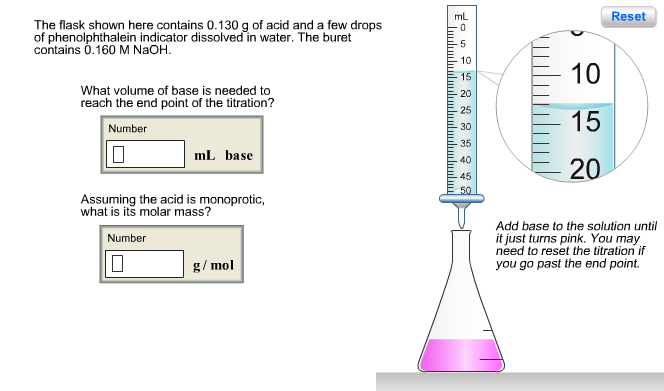
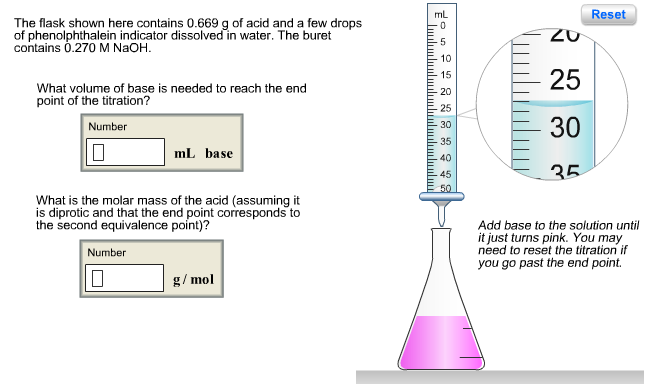
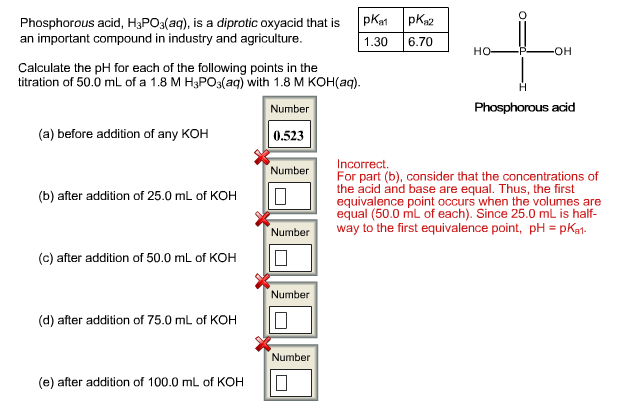
The flask shown here contains 0.130 g of acid and a few drops of phenolphthalein indicator dissolved in water. The buret contains 0.160 M NaOH. What volume of base is needed to reach the end point of the titration? Number 0 mL base Assuming the acid is monoprotic, what is its molar mass? Number 0 g/mol mL 5 10 15 -20 25 30 35 40 45 50 10 15 20 Reset Add base to the solution until it just turns pink. You may need to reset the titration if you go past the end point. The flask shown here contains 0.669 g of acid and a few drops of phenolphthalein indicator dissolved in water. The buret contains 0.270 M NaOH. What volume of base is needed to reach the end point of the titration? Number 10 mL base What is the molar mass of the acid (assuming it is diprotic and that the end point corresponds to the second equivalence point)? Number 0 g/mol mL 10 15 -20 -25 30 -35 40 -45 50 ZU 25 30 35 Reset Add base to the solution until it just turns pink. You may need to reset the titration if you go past the end point. Phosphorous acid, H3PO3(aq), is a diprotic oxyacid that is an important compound in industry and agriculture. Calculate the pH for each of the following points in the titration of 50.0 mL of a 1.8 M H3PO3(aq) with 1.8 M KOH(aq). Number 0.523 (a) before addition of any KOH (b) after addition of 25.0 mL of KOH (c) after addition of 50.0 mL of KOH (d) after addition of 75.0 mL of KOH (e) after addition of 100.0 mL of KOH Number 10 Number 10 Number Number pka1 pka2 1.30 6.70 H -OH Phosphorous acid Incorrect. For part (b), consider that the concentrations of the acid and base are equal. Thus, the first equivalence point occurs when the volumes are equal (50.0 mL of each). Since 25.0 mL is half- way to the first equivalence point, pH = pka1-
Step by Step Solution
3.66 Rating (190 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


