Question: Cp/R = 2.95 + .00495T (K) (3) Carbon dioxide is expanded through an adiabatic valve in steady flow from 400 K and 25 bars to
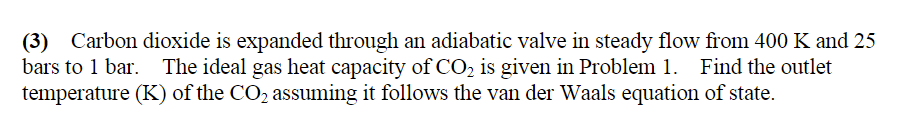
Cp/R = 2.95 + .00495T (K)
(3) Carbon dioxide is expanded through an adiabatic valve in steady flow from 400 K and 25 bars to 1 bar. The ideal gas heat capacity of CO2 is given in Problem 1. Find the outlet temperature (K) of the CO2 assuming it follows the van der Waals equation of state
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


