Question: Create a graph similar to the one provided. #5. (30%) Analyze the NOx kinetics by solving the Zeldovich mechanism problem. Based on #4 above, the

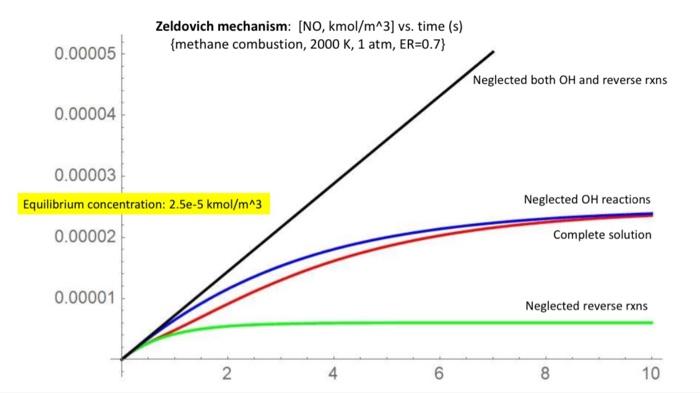
#5. (30%) Analyze the NOx kinetics by solving the Zeldovich mechanism problem. Based on #4 above, the most important graph is that of NO concentration vs. time. Right? Zeldovich mechanism: [NO, kmol/m^3) vs. time (s) {methane combustion, 2000K, 1 atm, ER=0.7} 0.00005 Neglected both OH and reverse rxns 0.00004 0.00003 Equilibrium concentration: 2.5e-5 kmol/m^3 0.00002 Neglected OH reactions Complete solution 0.00001 Neglected reverse rxns 2 4 6 8 10 #5. (30%) Analyze the NOx kinetics by solving the Zeldovich mechanism problem. Based on #4 above, the most important graph is that of NO concentration vs. time. Right? Zeldovich mechanism: [NO, kmol/m^3) vs. time (s) {methane combustion, 2000K, 1 atm, ER=0.7} 0.00005 Neglected both OH and reverse rxns 0.00004 0.00003 Equilibrium concentration: 2.5e-5 kmol/m^3 0.00002 Neglected OH reactions Complete solution 0.00001 Neglected reverse rxns 2 4 6 8 10
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


