Question: An ideal gas (the weight is m kg) in an idealized piston-cylinder assembly undergoes a series of processes from state 1 to states 2,
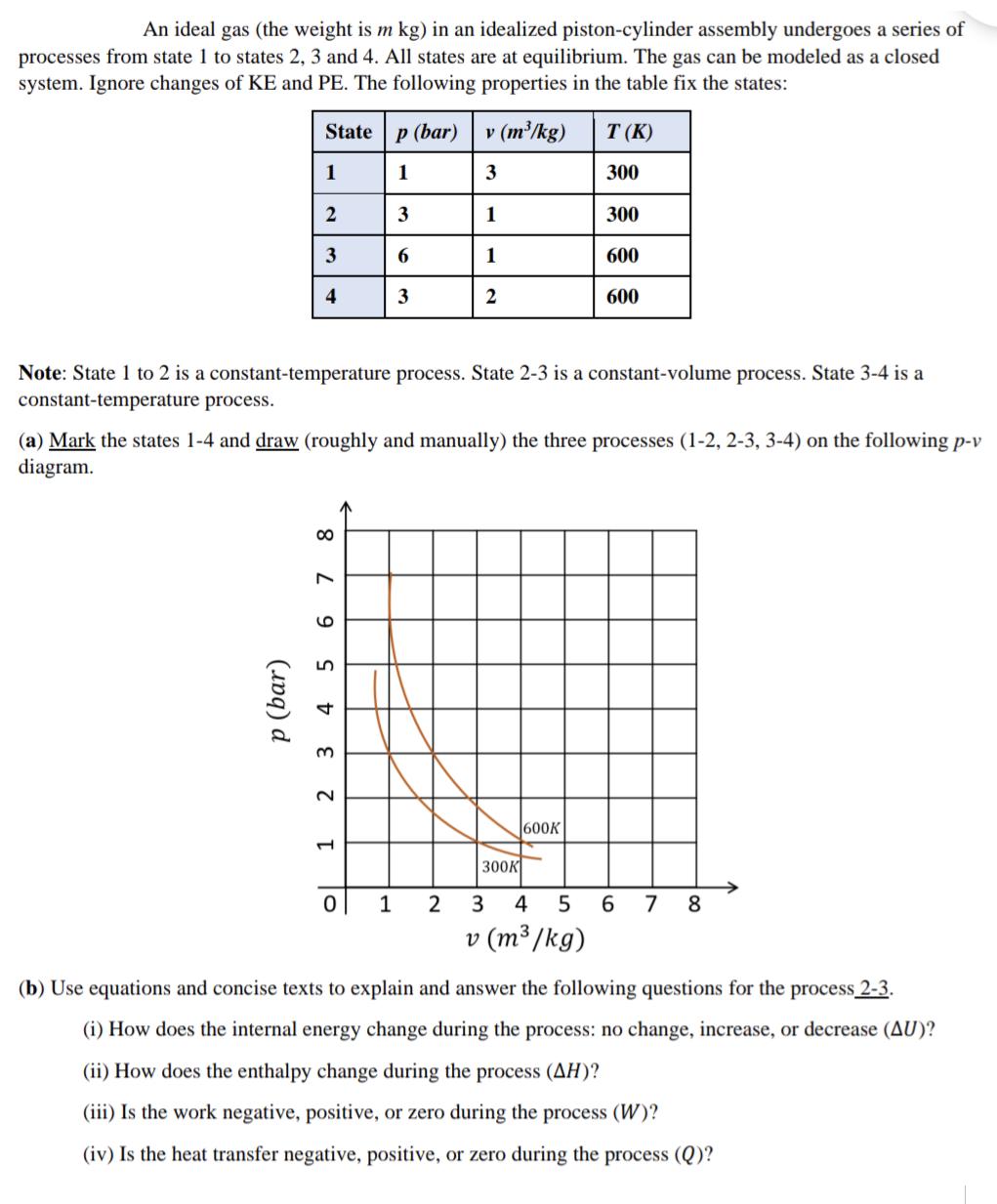
An ideal gas (the weight is m kg) in an idealized piston-cylinder assembly undergoes a series of processes from state 1 to states 2, 3 and 4. All states are at equilibrium. The gas can be modeled as a closed system. Ignore changes of KE and PE. The following properties in the table fix the states: State 1 p (bar) 2 3 4 8 6 7 5 tz 3 2 1 Note: State 1 to 2 is a constant-temperature process. State 2-3 is a constant-volume process. State 3-4 is a constant-temperature process. Ol p (bar) 1 (a) Mark the states 1-4 and draw (roughly and manually) the three processes (1-2, 2-3, 3-4) on the following p-v diagram. 0 3 6 1 3 v (m/kg) 3 1 1 2 2 300K T (K) 300 600K 300 3 4 5 v (m/kg) 600 600 6 7 8 (b) Use equations and concise texts to explain and answer the following questions for the process 2-3. (i) How does the internal energy change during the process: no change, increase, or decrease (AU)? (ii) How does the enthalpy change during the process (AH)? (iii) Is the work negative, positive, or zero during the process (W)? (iv) Is the heat transfer negative, positive, or zero during the process (Q)?
Step by Step Solution
3.45 Rating (148 Votes )
There are 3 Steps involved in it
St... View full answer

Get step-by-step solutions from verified subject matter experts


