Question: 1) Consider the reaction energy diagram shown below and answer the associated questions. Overall Reaction: X Y + Z Rate Law: rate= K[X] Enthalpy
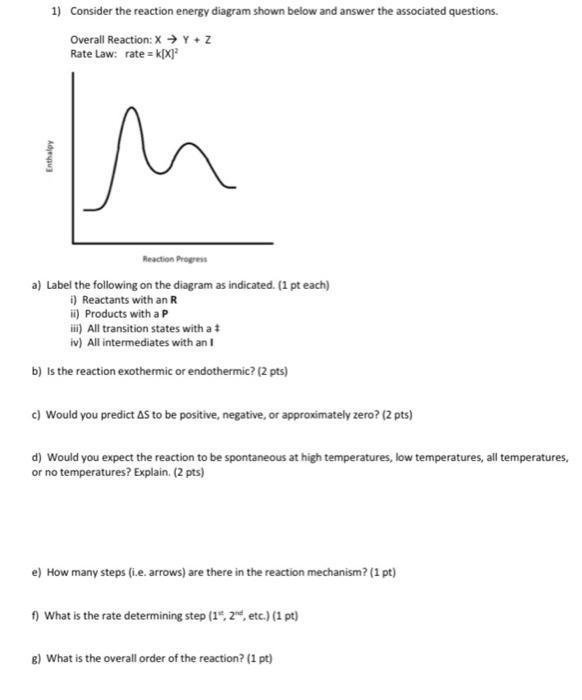
1) Consider the reaction energy diagram shown below and answer the associated questions. Overall Reaction: X Y + Z Rate Law: rate= K[X] Enthalpy Reaction Progress a) Label the following on the diagram as indicated. (1 pt each) i) Reactants with an R ii) Products with a P iii) All transition states with a t iv) All intermediates with an I b) is the reaction exothermic or endothermic? (2 pts) c) Would you predict AS to be positive, negative, or approximately zero? (2 pts) d) Would you expect the reaction to be spontaneous at high temperatures, low temperatures, all temperatures, or no temperatures? Explain. (2 pts) e) How many steps (i.e. arrows) are there in the reaction mechanism? (1 pt) f) What is the rate determining step (1, 2, etc.) (1 pt) g) What is the overall order of the reaction? (1 pt)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


