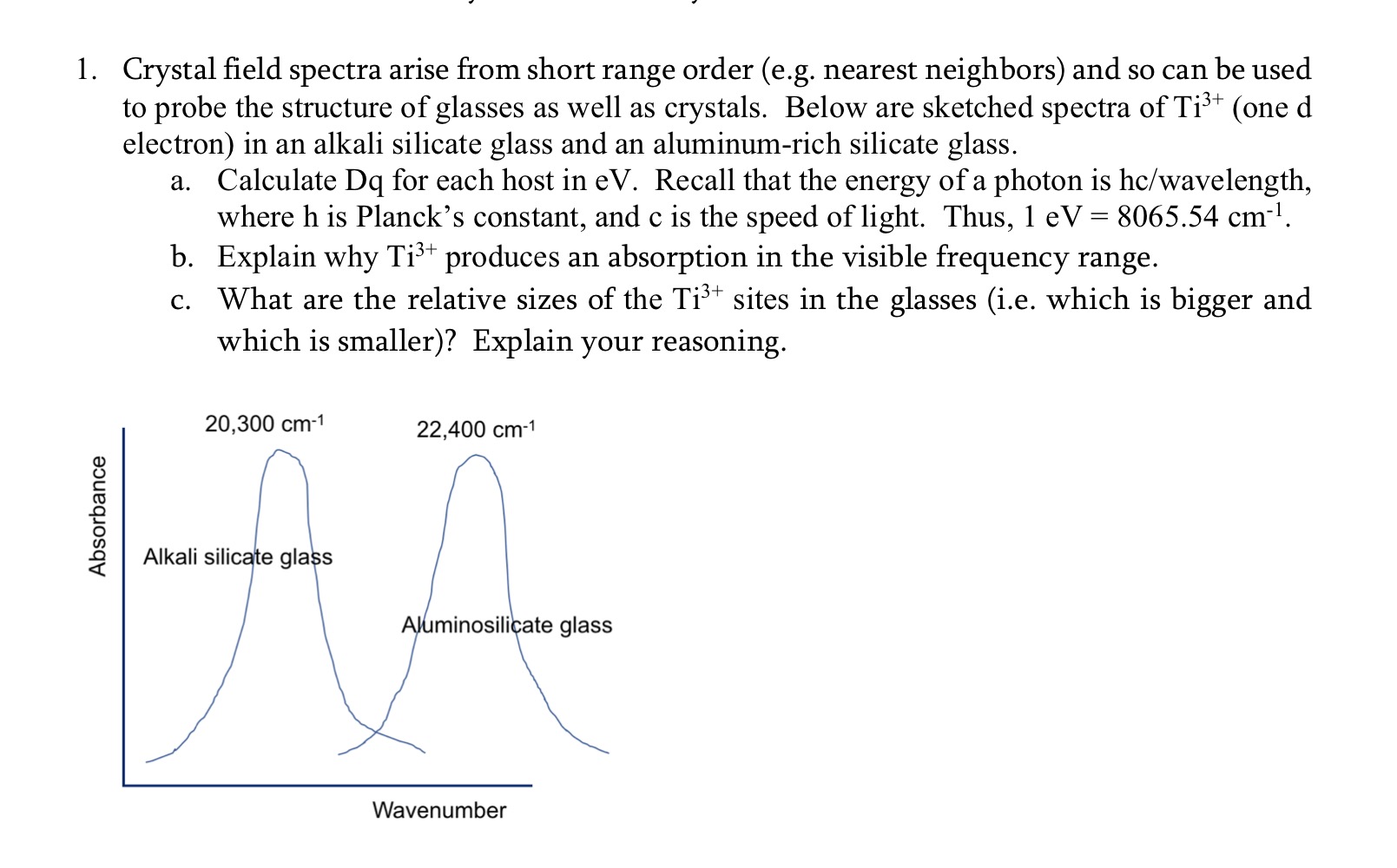
Question: Crystal field spectra arise from short range order ( e . g . nearest neighbors ) and so can be used to probe the structure
Crystal field spectra arise from short range order eg nearest neighbors and so can be used to probe the structure of glasses as well as crystals. Below are sketched spectra of Tione d electron in an alkali silicate glass and an aluminumrich silicate glass.
a Calculate Dq for each host in eV Recall that the energy of a photon is hcwavelength where h is Plancks constant, and c is the speed of light. Thus, eV cm
b Explain why Ti produces an absorption in the visible frequency range.
c What are the relative sizes of the Ti sites in the glasses ie which is bigger and
which is smaller Explain your reasoning.Crystal field spectra arise from short range order eg nearest neighbors and so can be used
to probe the structure of glasses as well as crystals. Below are sketched spectra of one
electron in an alkali silicate glass and an aluminumrich silicate glass.
a Calculate Dq for each host in Recall that the energy of a photon is hcwavelength
where is Planck's constant, and is the speed of light. Thus,
b Explain why produces an absorption in the visible frequency range.
c What are the relative sizes of the sites in the glasses ie which is bigger and
which is smaller Explain your reasoning.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


