Question: Cu2S+O2=2Cu+SO2 Copper scrap is added to help cool the process. Typically, 200kg of pure copper scrap is added per total tonne of copper produced. The
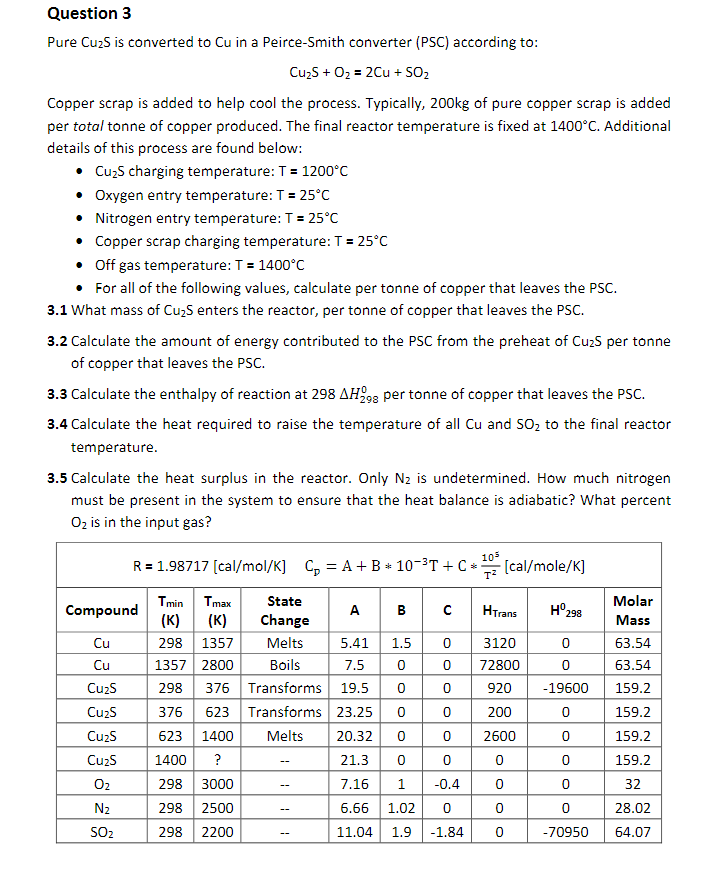
Cu2S+O2=2Cu+SO2 Copper scrap is added to help cool the process. Typically, 200kg of pure copper scrap is added per total tonne of copper produced. The final reactor temperature is fixed at 1400C. Additional details of this process are found below: - Cu2S charging temperature: T=1200C - Oxygen entry temperature: T=25C - Nitrogen entry temperature: T=25C - Copper scrap charging temperature: T=25C - Off gas temperature: T=1400C - For all of the following values, calculate per tonne of copper that leaves the PSC. 3.1 What mass of Cu2S enters the reactor, per tonne of copper that leaves the PSC. 3.2 Calculate the amount of energy contributed to the PSC from the preheat of Cu2S per tonne of copper that leaves the PSC. 3.3 Calculate the enthalpy of reaction at 298H2980 per tonne of copper that leaves the PSC. 3.4 Calculate the heat required to raise the temperature of all Cu and SO2 to the final reactor temperature. 3.5 Calculate the heat surplus in the reactor. Only N2 is undetermined. How much nitrogen must be present in the system to ensure that the heat balance is adiabatic? What percent O2 is in the input gas? R=198717[raa/mal/K1C=A+R103T+Cr105ral/malo/K1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


