Question: Current Attempt in Progress An aqueous solution containing 0.924g of a sugar in 100mL of a solution has an osmotic pressure of 1.26 bar at
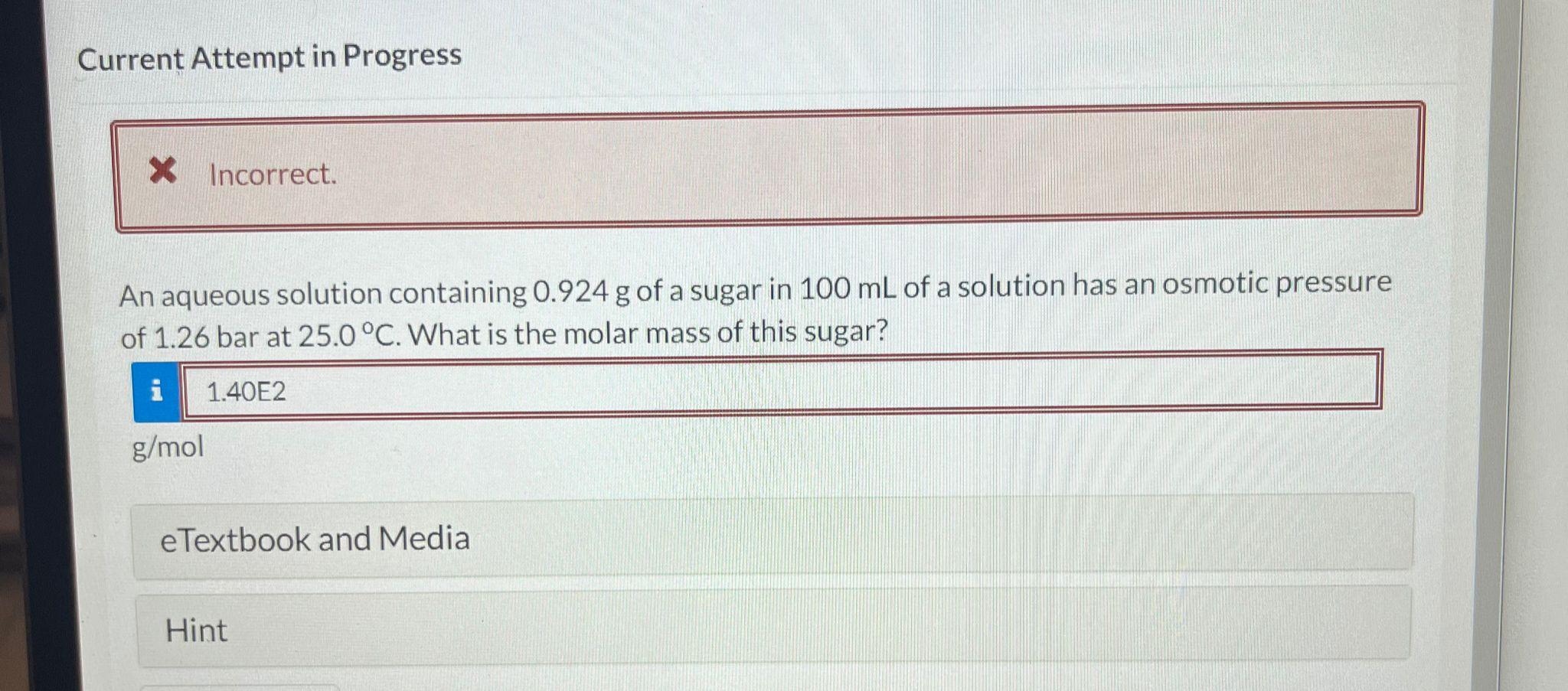

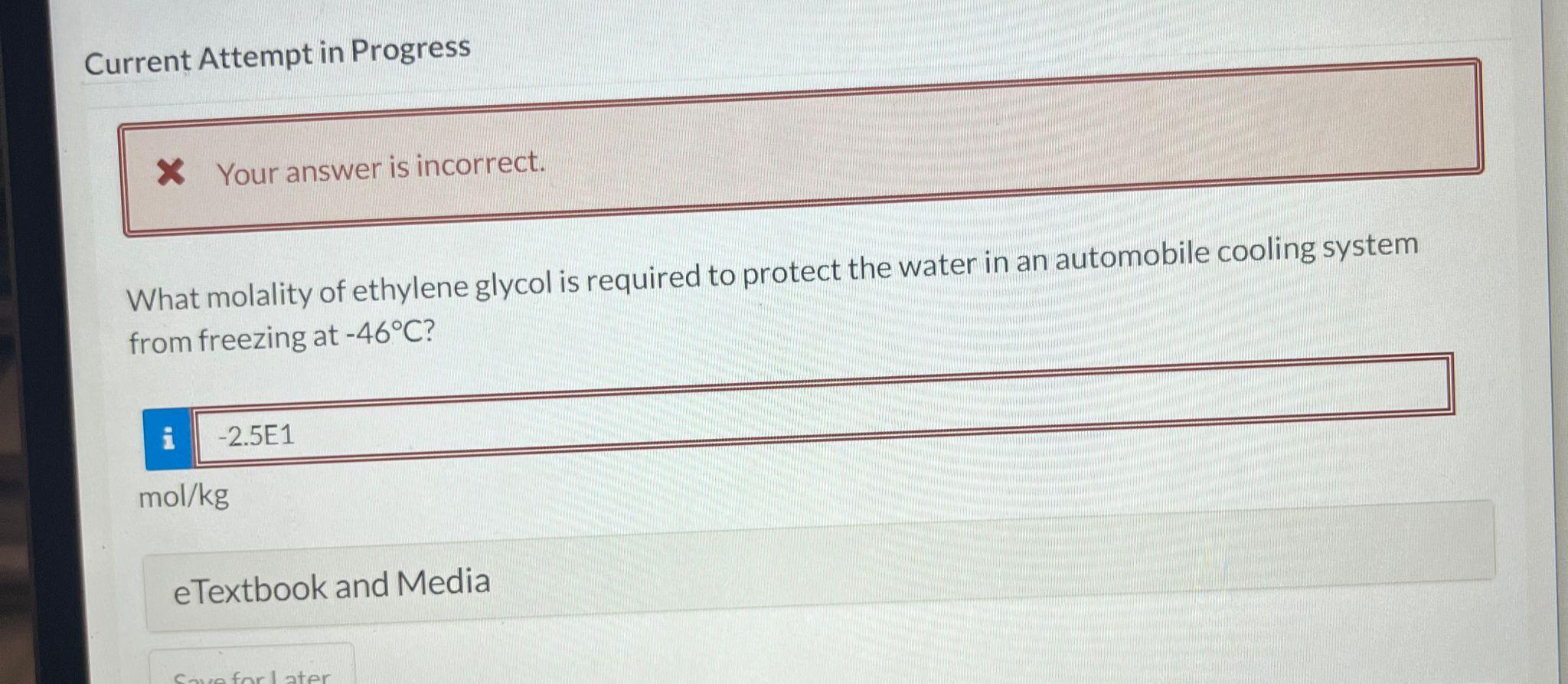
Current Attempt in Progress An aqueous solution containing 0.924g of a sugar in 100mL of a solution has an osmotic pressure of 1.26 bar at 25.0C. What is the molar mass of this sugar? Current Attempt in Progress The freezing point of an aqueous solution containing pantothenic acid (M=205.3g/mol) is 0.62C. Calculate the osmotic pressure at 18C, at which the density of the solution is 1.012g/mL. Assume pantothenic acid not dissociating in solution. Current Attempt in Progress What molality of ethylene glycol is required to protect the water in an automobile cooling system from freezing at 46C
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


