Question: Current Attempt in Progress Consider a hypothetical metal that has a density of 16.4 g/cm2, an atomic weight of 193.8 g/mol, and an atomic radius
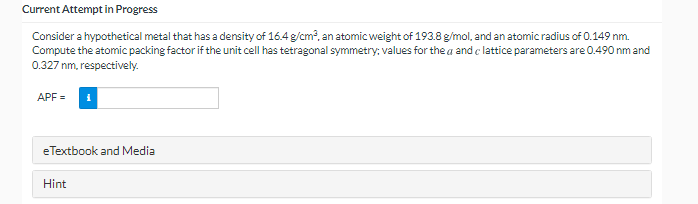
Current Attempt in Progress Consider a hypothetical metal that has a density of 16.4 g/cm2, an atomic weight of 193.8 g/mol, and an atomic radius of 0.149 nm. Compute the atomic packing factor if the unit cell has tetragonal symmetry; values for the a and clattice parameters are 0.490 nm and 0.327 nm, respectively. APF = eTextbook and Media Hint
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


