Question: Current Attempt in Progress Hydrofluoric acid dissolves glass and must be stored in plastic containers. What mass of a 19 %/w/w) solution of HF(aq) contains
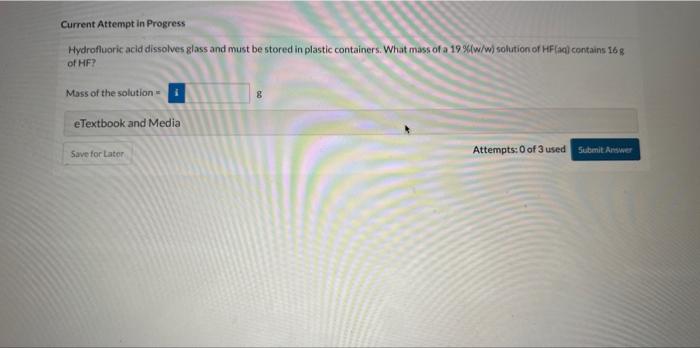
Current Attempt in Progress Hydrofluoric acid dissolves glass and must be stored in plastic containers. What mass of a 19 \%/w/w) solution of HF(aq) contains i6 g of HF? Mass of the solution = g eTextbook and Media Attempts: 0 of 3 used
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


