Question: Current Attempt in Progress Synthetically produced ethanol is an important industrial commodity used for various purposes including: as a solvent (especially for substances intended for
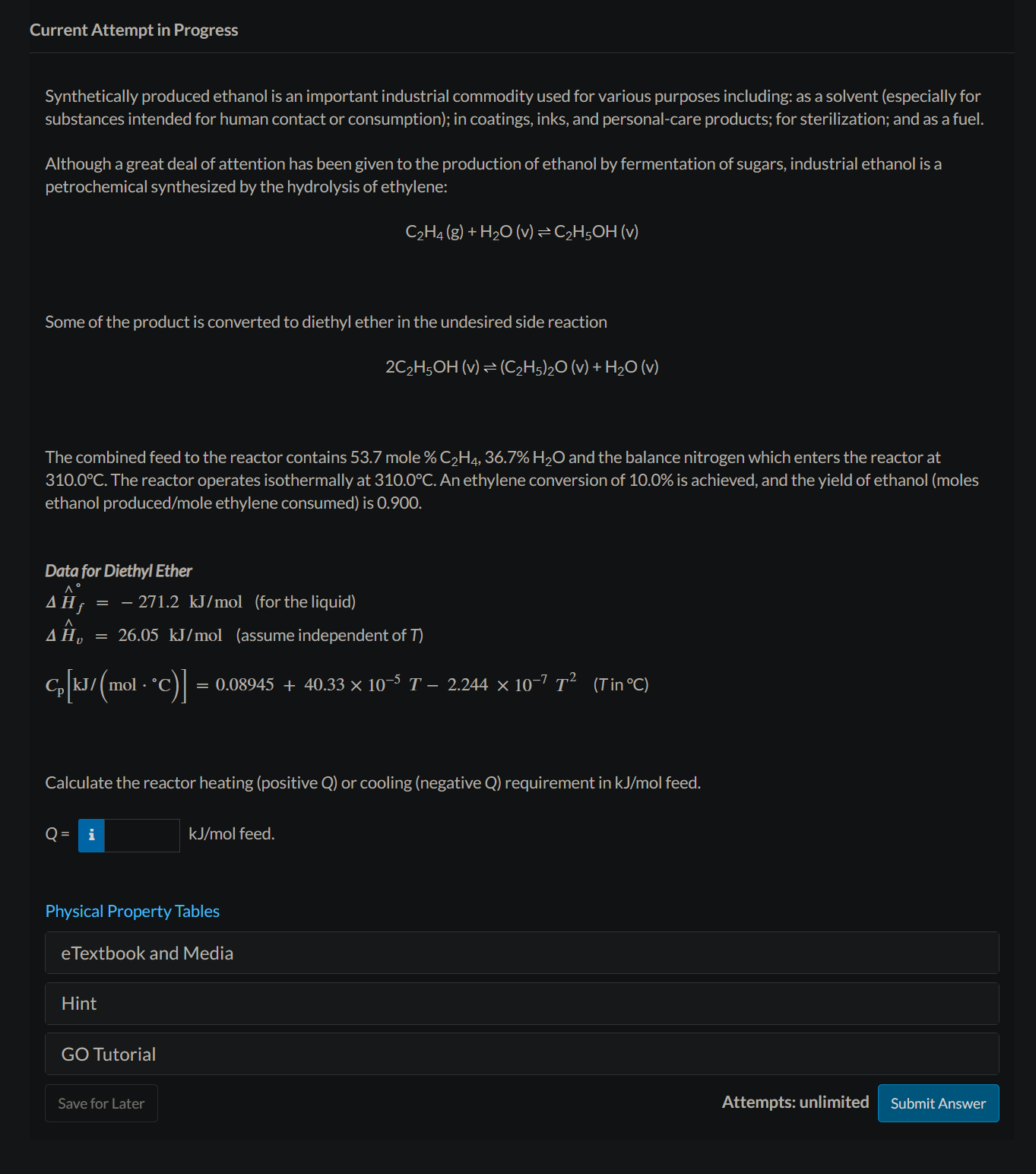
Current Attempt in Progress Synthetically produced ethanol is an important industrial commodity used for various purposes including: as a solvent (especially for substances intended for human contact or consumption); in coatings, inks, and personal-care products; for sterilization; and as a fuel. Although a great deal of attention has been given to the production of ethanol by fermentation of sugars, industrial ethanol is a petrochemical synthesized by the hydrolysis of ethylene: C2H4(g)+H2O(v)C2H5OH(v) Some of the product is converted to diethyl ether in the undesired side reaction 2C2H5OH(v)(C2H5)2O(v)+H2O(v) The combined feed to the reactor contains 53.7 mole %C2H4,36.7%H2O and the balance nitrogen which enters the reactor at 310.0C. The reactor operates isothermally at 310.0C. An ethylene conversion of 10.0% is achieved, and the yield of ethanol (moles ethanol produced/mole ethylene consumed) is 0.900. Data for Diethyl Ether H^f=271.2kJ/mol(fortheliquid)H^v=26.05kJ/mol(assumeindependentofT)Cp[kJ/(molC)]=0.08945+40.33105T2.244107T2(TinC) Calculate the reactor heating (positive Q ) or cooling (negative Q ) requirement in kJ/mol feed. Q= kJ/mol feed. Physical Property Tables eTextbook and Media Hint GO Tutorial
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


