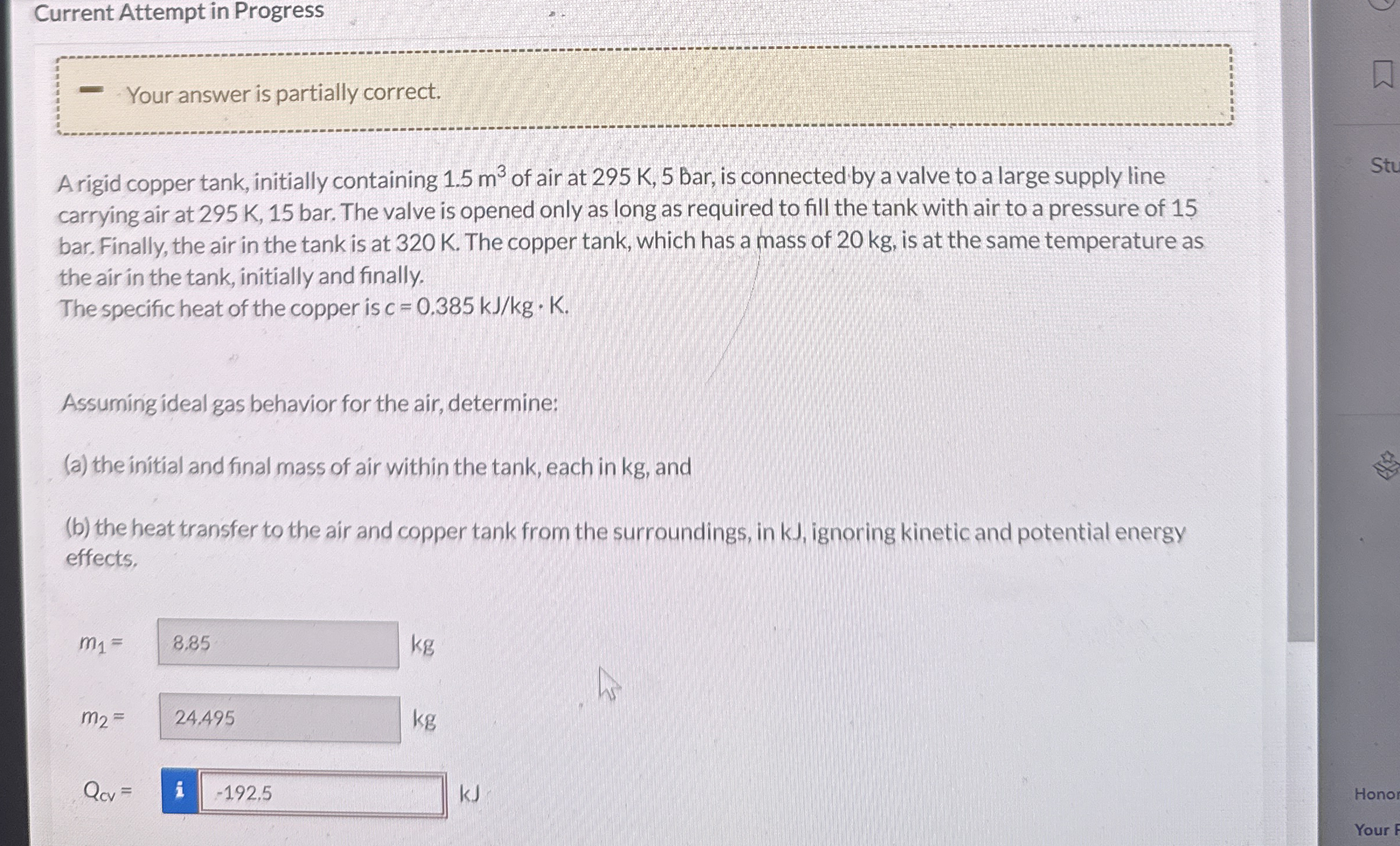
Question: Current Attempt in Progress Your answer is partially correct. A rigid copper tank, initially containing 1 . 5 m 3 of air at 2 9
Current Attempt in Progress
Your answer is partially correct.
A rigid copper tank, initially containing of air at is connected by a valve to a large supply line carrying air at bar. The valve is opened only as long as required to fill the tank with air to a pressure of bar. Finally, the air in the tank is at K The copper tank, which has a mass of kg is at the same temperature as the air in the tank, initially and finally.
The specific heat of the copper is
Assuming ideal gas behavior for the air, determine:
a the initial and final mass of air within the tank, each in kg and
b the heat transfer to the air and copper tank from the surroundings, in kJ ignoring kinetic and potential energy effects.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


