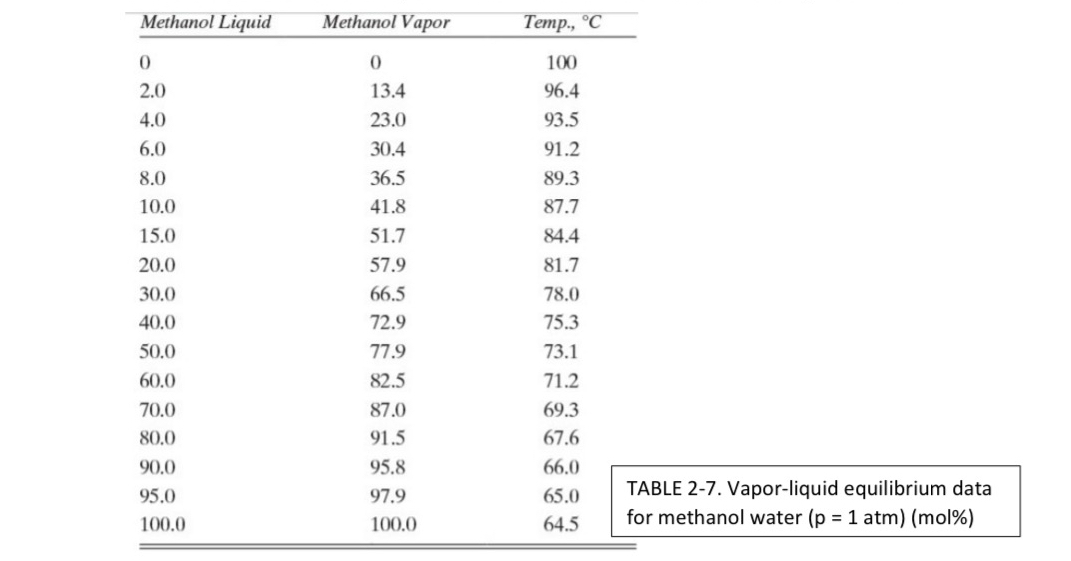
Question: D 1 . * We are separating a mixture of methanol and water in a flash drum at 1 . 0 atm pressure. Equilibrium data
D We are separating a mixture of methanol and water in a flash drum at atm pressure. Equilibrium data are listed in Table
a Feed is mol methanol, and of the feed is vaporized. What are the vapor and liquid mole fractions and flow rates? Feed rate is kmo
b Repeat part a for a feed rate of kmo
c If the feed is mol methanol and we desire a liquid product that is mol methanol, what VF must be used? For a feed rate of lbmo find product flow rates and compositions.
d We are operating the flash drum so that the liquid mole fraction is mol methanol. kmo and What must the flow rate and composition of the feed be
e Find the dimensions of a vertical flash drum for Problem Dc
Data: Assume vapors are ideal gas.
f If atm, and Tdrum find and
g If atm, and mole fraction methanol, find and
tableMethanol Liquid,Methanol Vapor,Temp., TABLE Vaporliquid equilibrium datafor methanol water atm
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


