Question: d. 7 protons, 7 electrons e. 34 protons, 46 neutrons and 36 electrons 7. (5pts) Consider 23Na,88Sr2+,Se,Br,35Cl. a. Which of these are ions? 88Sr2+,Br b.
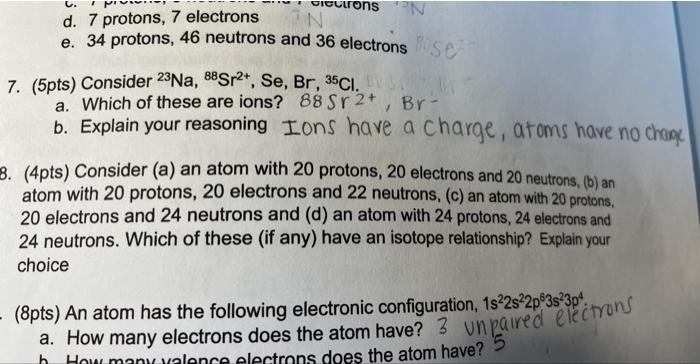
d. 7 protons, 7 electrons e. 34 protons, 46 neutrons and 36 electrons 7. (5pts) Consider 23Na,88Sr2+,Se,Br,35Cl. a. Which of these are ions? 88Sr2+,Br b. Explain your reasoning Ions have a charge, atoms have no chang 3. (4pts) Consider (a) an atom with 20 protons, 20 electrons and 20 neutrons, (b) an atom with 20 protons, 20 electrons and 22 neutrons, (c) an atom with 20 protons. 20 electrons and 24 neutrons and (d) an atom with 24 protons, 24 electrons and 24 neutrons. Which of these (if any) have an isotope relationship? Explain your choice (8pts) An atom has the following electronic configuration, 1s22s22p63s23p4 a. How many electrons does the atom have? 3 un paired electrons
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


