Question: d) A 0.3396-g sample that assayed 96.4%Na2SO4 was titrated with a solution of BaCl2 : Ba2++SO42BaSO4(s) Calculate the molarity of the BaCl2 solution if the
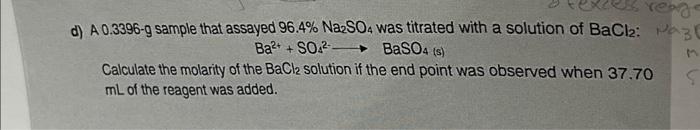
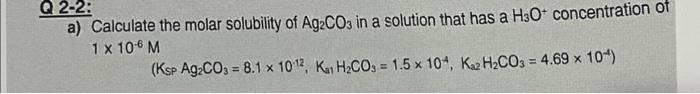
d) A 0.3396-g sample that assayed 96.4%Na2SO4 was titrated with a solution of BaCl2 : Ba2++SO42BaSO4(s) Calculate the molarity of the BaCl2 solution if the end point was observed when 37.70 mL of the reagent was added. a) Calculate the molar solubility of Ag2CO3 in a solution that has H3O+concentration of 1106M (KspAg2CO3=8.11012,Ka12H2CO3=1.5104,K22H2CO3=4.69104)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


