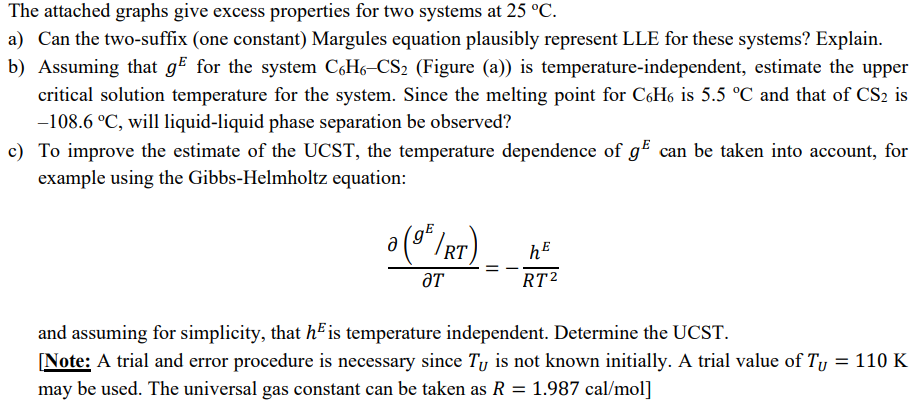
Question: d e l ( g E R T ) d e l T = - h E R T 2 and assuming for simplicity, that
and assuming for simplicity, that is temperature independent. Determine the UCST.
Note: A trial and error procedure is necessary since is not known initially. A trial value of
may be used. The universal gas constant can be taken as
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


