Question: (d) In 1925, George E. Briggs and John B. S. Haldane applied the steady-state approximation method to determine the rate law of the enzyme-catalyzed reaction:
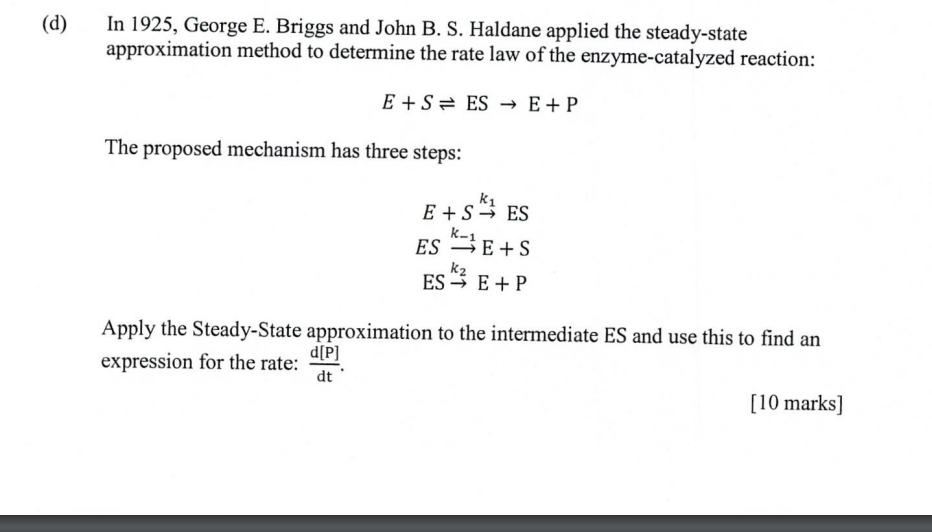
(d) In 1925, George E. Briggs and John B. S. Haldane applied the steady-state approximation method to determine the rate law of the enzyme-catalyzed reaction: E+S= ES E+P The proposed mechanism has three steps: ki E +S ES S4 ES $E+S ES"} E+ P k2 Apply the Steady-state approximation to the intermediate ES and use this to find an d[P] expression for the rate: dt [10 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


