Question: D please 1) Prove the listed value, the calculated value using Gf and the calculated value using Hf and Sf values agree for the following
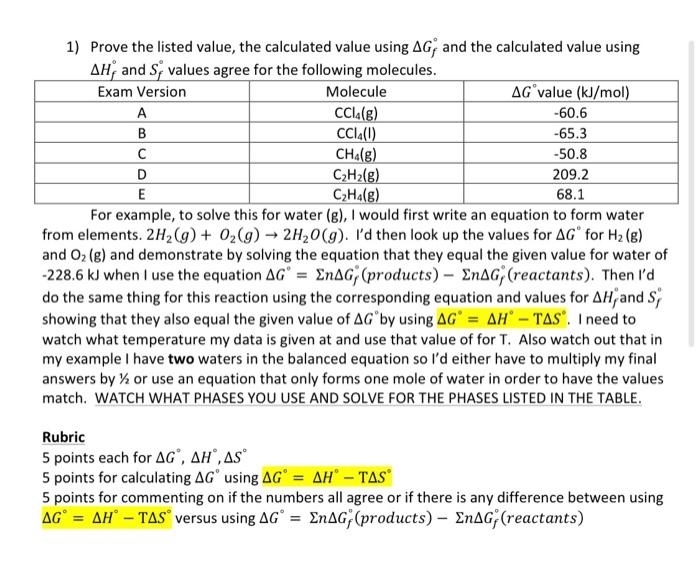
1) Prove the listed value, the calculated value using Gf and the calculated value using Hf and Sf values agree for the following molecules. For example, to solve this for water (g), I would first write an equation to form water from elements. 2H2(g)+O2(g)2H2O(g). I'd then look up the values for G for H2(g) and O2(g) and demonstrate by solving the equation that they equal the given value for water of 228.6kJ when I use the equation G=nGf (products) nGf (reactants). Then I'd do the same thing for this reaction using the corresponding equation and values for Hf and Sf showing that they also equal the given value of G by using G=HTS. I need to watch what temperature my data is given at and use that value of for T. Also watch out that in my example I have two waters in the balanced equation so I'd either have to multiply my final answers by 1/2 or use an equation that only forms one mole of water in order to have the values match. WATCH WHAT PHASES YOU USE AND SOLVE FOR THE PHASES LISTED IN THE TABLE. Rubric 5 points each for G,H,S 5 points for calculating G using G=HTS 5 points for commenting on if the numbers all agree or if there is any difference between using G=HTS versus using G=nGf( products )nGf( reactants )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


