Question: d S = ( d e l S d e l T ) V d T + ( d e l S d e l
please find for mole of ideal gas changes from and to and where olK, and the state function of ideal gas is hint: you should use one of Maxwell relations to solve this calculation.
Integrals : ab
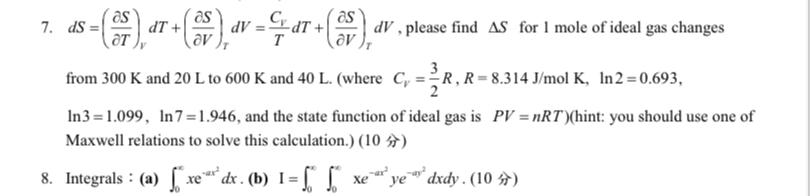
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


